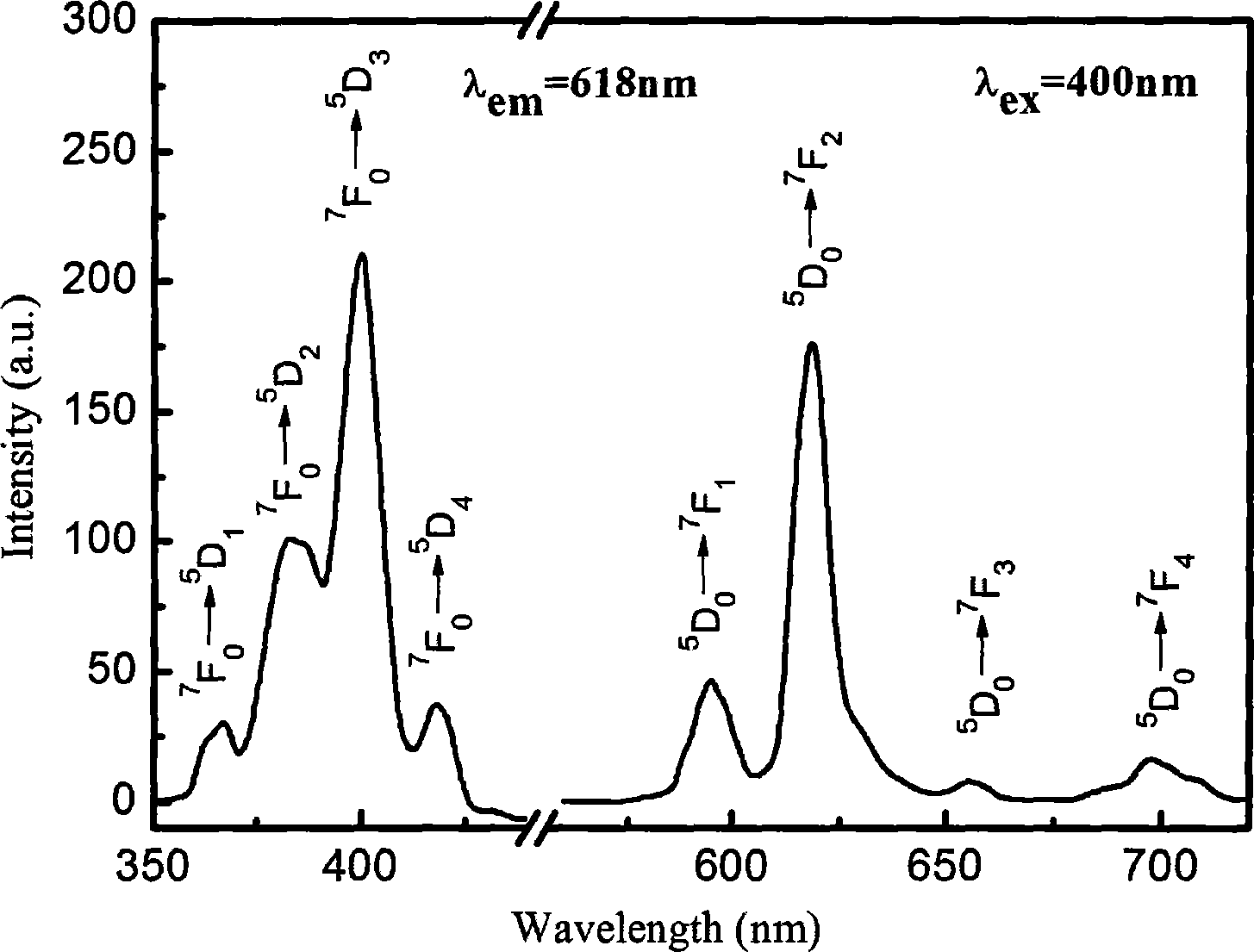
Preparation of doped calcium titanate (CaTiO3: Eu3+) fluorescent powder
A technology of fluorescent powder and calcium titanate, applied in chemical instruments and methods, titanium compounds, luminescent materials, etc., can solve the problems of low luminous efficiency and achieve high chemical stability, simple process, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Weigh 9.912g of calcium nitrate tetrahydrate and 3.568g of europium nitrate hexahydrate, add them into a beaker, and then add 100ml of absolute ethanol; weigh 17.000g of n-tetrabutyl titanate, add them into a beaker, and then add 100ml of absolute ethanol. Stir the above two mixtures for 30 minutes to form a solution; add the mixed ethanol solution of the above-mentioned europium salt and calcium salt to the n-tetrabutyl titanate ethanol solution, and stir for 8 minutes at the same time to form a sol; place the sol at a temperature of 5°C for 10 minutes, To obtain a wet gel, put the wet gel into an oven at 50°C and dry it for 15 hours to obtain a dry gel; finally, calcinate the obtained precursor at 1400°C for 2 hours to obtain europium-doped calcium titanate CaTiO 3 :Eu 3+ Phosphor.
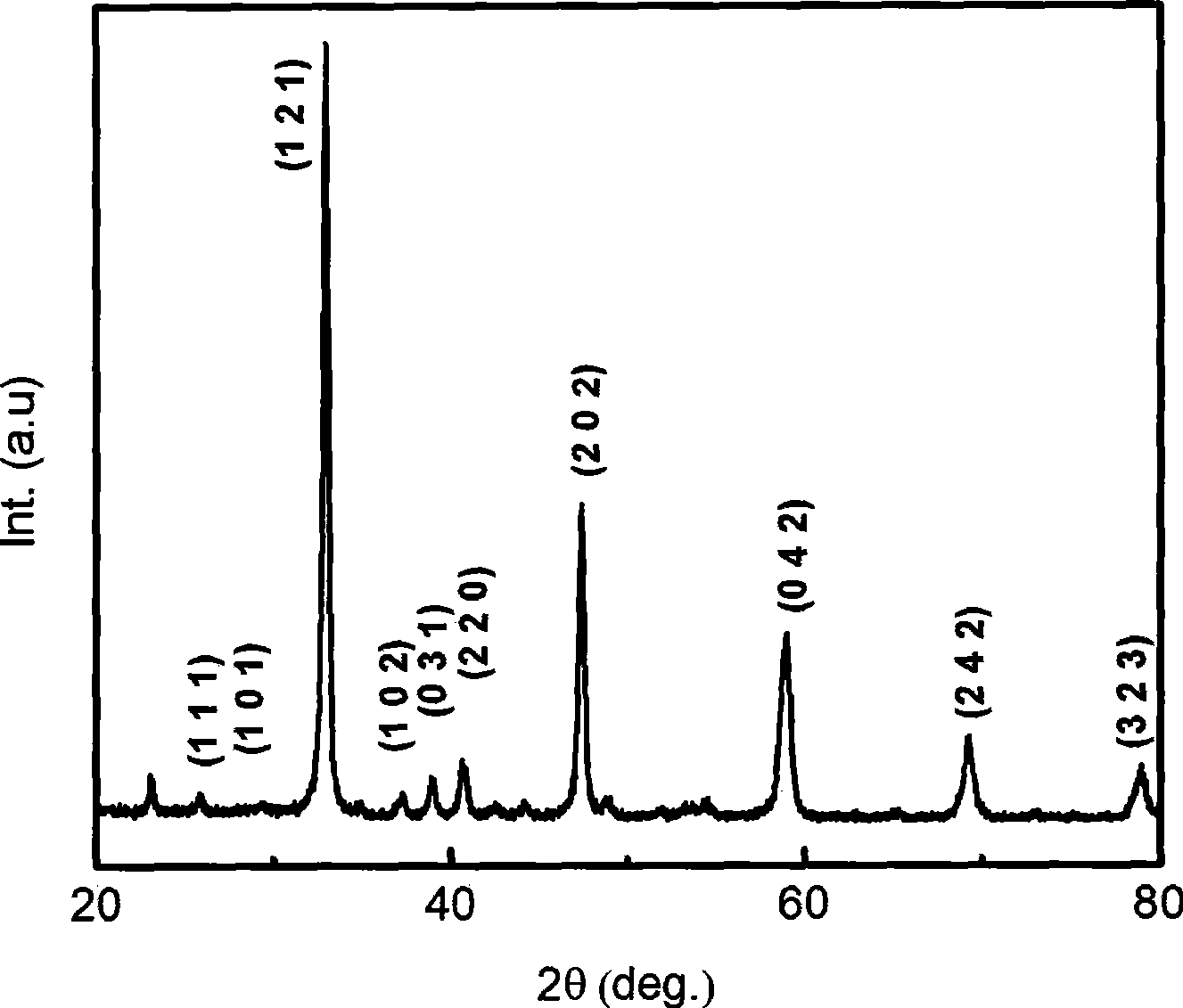
[0018] figure 1 It is the X-ray diffraction pattern of the fluorescent powder synthesized in this embodiment, it can be seen that the diffraction peaks are consistent with the diffracti...
Embodiment 2
[0022] Weigh 9.676g of calcium nitrate tetrahydrate and 4.014g of europium nitrate hexahydrate, add them into a beaker, and then add 50ml of absolute ethanol; weigh 17.000g of n-tetrabutyl titanate, add them into a beaker, and then add 50ml of absolute ethanol. Stir the above two mixtures for 20 minutes to form a solution; add the mixed ethanol solution of the above-mentioned europium salt and calcium salt to the n-tetrabutyl titanate ethanol solution, and stir for 10 minutes at the same time to form a sol; place the sol at 10°C for 20 minutes, To obtain a wet gel, put the wet gel into an oven at 120°C and dry it for 18 hours to obtain a dry gel; finally, calcinate the obtained precursor at 1300°C for 4 hours to obtain europium-doped calcium titanate CaTiO 3 :Eu 3+ Phosphor.
[0023] The X-ray test results show that the diffraction peaks are consistent with the diffraction peaks of calcium titanate in the cubic phase, and no diffraction peaks belonging to other oxides of calc...
Embodiment 3
[0027] Weigh 9.440g of calcium nitrate tetrahydrate and 4.460g of europium nitrate hexahydrate, add them into a beaker, and then add 60ml of absolute ethanol; weigh 17.000g of n-tetrabutyl titanate, add them into a beaker, and then add 60ml of absolute ethanol. Stir the above two mixtures for 25 minutes to form a solution; add the mixed ethanol solution of the above-mentioned europium salt and calcium salt to the n-tetrabutyl titanate ethanol solution, and stir for 12 minutes at the same time to form a sol; place the sol at 20°C for 30 minutes, To obtain a wet gel, put the wet gel into an oven at 80°C and dry it for 20 hours to obtain a dry gel; finally, calcinate the obtained precursor at 1400°C for 6 hours to obtain europium-doped calcium titanate CaTiO 3 :Eu 3+ Phosphor.
[0028] The X-ray test results show that the diffraction peaks are consistent with the diffraction peaks of calcium titanate in the cubic phase, and no diffraction peaks belonging to other oxides of calci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com