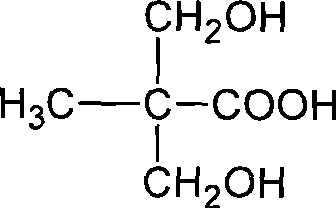
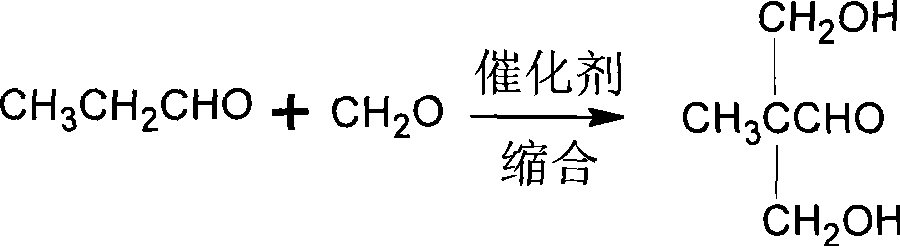
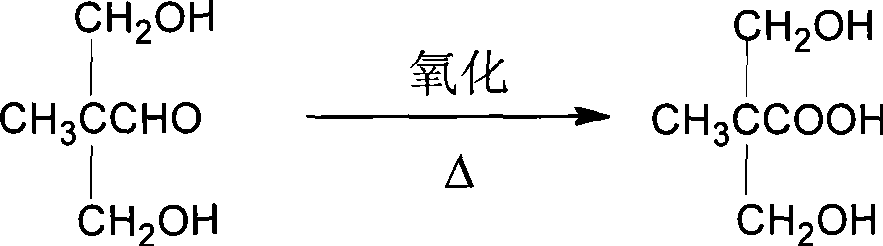
Method for synthesizing 2,2-bis(hydroxymenthyl)propinonic acid
A technology of dimethylolpropionic acid and synthesis method, applied in the directions of oxidative preparation of carboxylic acid, organic chemistry, etc., can solve the problems such as decreased yield of 2,2 dimethylolpropionic acid, high content of by-products, etc. Low, high oxidation efficiency, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Add a certain amount of formaldehyde and Ba(OH) 2 , formaldehyde and Ba(OH) 2 The ratio of formaldehyde to propionaldehyde is 1:0.05, the mass ratio; take an appropriate amount of propionaldehyde in the dropping funnel, the ratio of formaldehyde and propionaldehyde is 2.2:1, the molar ratio; start stirring, control the pH at 10, and the temperature at 250 ° C, slowly Propionaldehyde was added dropwise, and the dropwise addition was completed, keeping the pH at 10 to continue the reaction for 4h.
[0063] Pour the prepared 2,2-dimethylolpropanal into a 250 ml four-necked bottle equipped with a stirrer, a reflux condenser, a dropping funnel and a thermometer, add 100 ml of distilled water for dilution, and take an appropriate amount of 30% over The hydrogen peroxide solution is placed in the dropping funnel, the molar ratio of hydrogen peroxide to propionaldehyde is 1:1; start stirring, and after the temperature rises to 60°C and stabilizes, slowly add hydrogen peroxide ...
Embodiment 2
[0065] Embodiment 2: the influence of the selection of condensation reaction catalyst on reaction
[0066] Other is the same as embodiment 1, changes the catalyzer of condensation reaction, and experimental result is shown in table 2.
[0067] The influence of the selection of table 2 condensation reaction catalyst on reaction
[0068]
condensation catalyst
Formaldehyde and Propionaldehyde
The molar ratio of Reaction time
(h) temperature reflex
(℃)
PH value
molar yield
(%) Triethylamine (5%) 2.2:1 4 25 10 43.2 NaOH(5%) 2.2:1 4 25 10 63.1 Ca(OH)2 2.2:1 4 25 10 67.6 Triethylamine(5%)+Ca(OH) 2 2.2:1 4 25 10 87.5 Ammonia (5%) 2.2:1 4 25 10 26.1 Triethylamine (5%)+Ba(OH) 2 2.2:1 4 25 10 88.2 Ba(OH) 2 2.2:1 4 25 10 70.1
[0069]
[0070] It can be seen from the experimental results that NaOH and Ca(OH) 2 、Ba(OH) 2 When used as a catalyst, the yie...
Embodiment 3
[0072] Add triethylamine and Ca(OH) 2 mixture of formaldehyde, triethylamine and Ca(OH) 2 The mixture: formaldehyde=0.02:1, molar ratio;
[0073] Propionaldehyde is added in the dropping funnel, the ratio of formaldehyde and propionaldehyde is 2.1:1, molar ratio;
[0074] Start stirring, control pH = 10, temperature at 20°C, slowly add propionaldehyde dropwise, keep the pH at 10 and continue the reaction for 4 hours;
[0075]After the reaction, add an appropriate amount of formic acid to neutralize to pH = 7, add the reaction materials to a round bottom flask, and carry out vacuum distillation (0.4 atmospheric pressure) to remove unreacted formaldehyde and propionaldehyde; use methyl isobutyl The ketone was extracted three times to remove the side reaction product methacrolein. The volume ratio of methyl isobutyl ketone to mother liquor is 1:1, and the extraction time is 10 minutes. After standing still, divide into two layers, discard the methyl isobutyl ketone phase, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com