Anarexol cream and preparation method thereof
A technology of cyproheptadine hydrochloride cream and cyproheptadine hydrochloride, applied in anti-inflammatory agents, ointment delivery, pharmaceutical formulations, etc., can solve the problem of novel appearance, insufficient unique effect, unsatisfactory clinical treatment, narrowing application range, etc. problems, to achieve the effect of good spiritual curative effect, unique administration method, and uniform content of the main drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
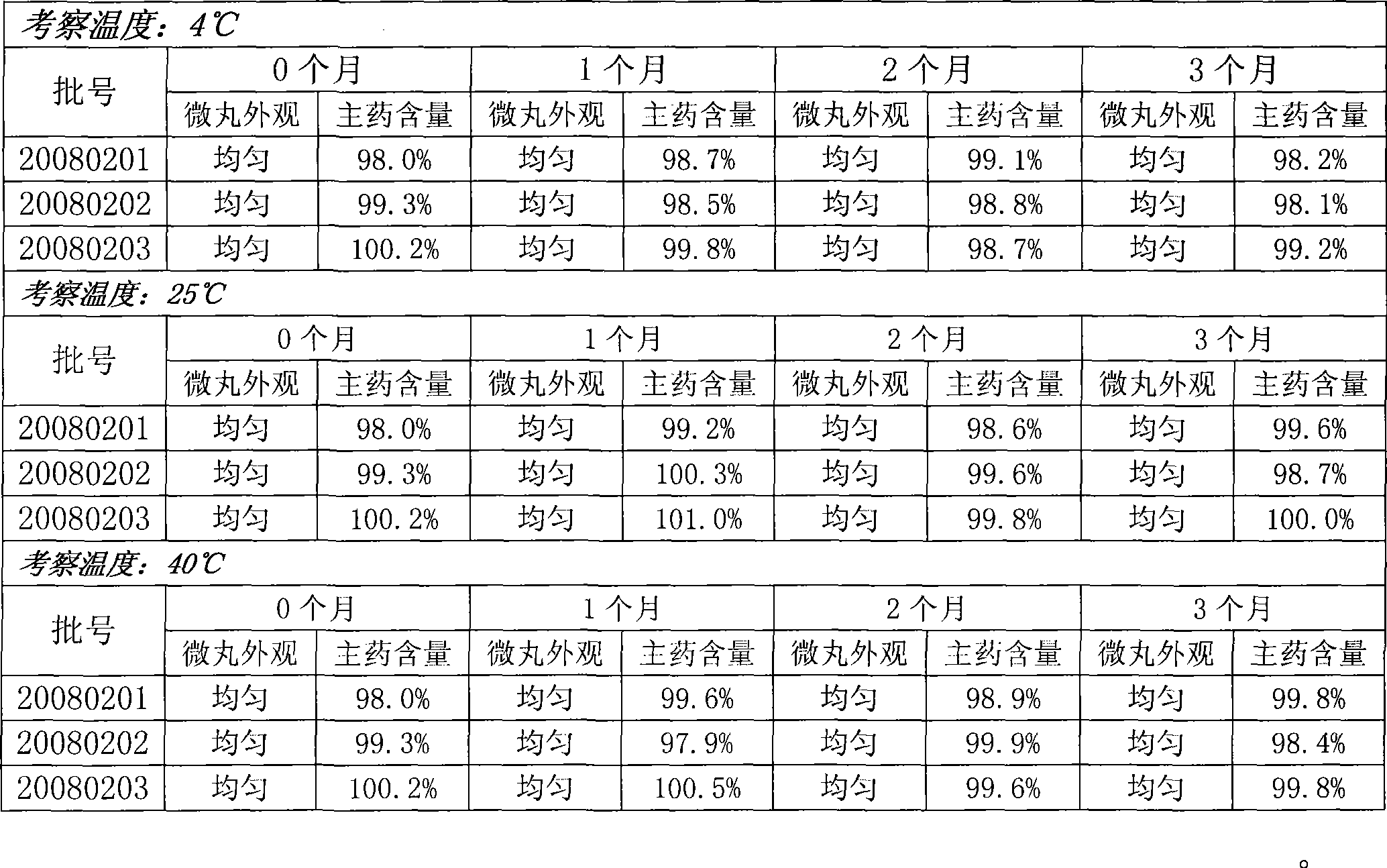
[0032] Example 1: batch number 20080201
[0033] Cyproheptadine Hydrochloride 55.5g
[0034] Mint pellets 200g
[0035] Stearic acid 825g
[0036] Glyceryl monostearate 515g
[0037] Triethanolamine 100g
[0038] White Vaseline 720g
[0039] Glycerin 720g
[0040] Ethylparaben 10g
[0041] Fragrance 10ml
[0042] Purified water 7500g
[0043] 1000 pieces
[0044] Preparation:
[0045] 1. Preparation of Peppermint Pellets:
[0046] (1) Glyceryl monostearate of 20 to 40 meshes is used as the core material of the skeleton ball;
[0047] (2) Take mint equal to the weight of the glyceryl monostearate ball core, and dissolve it in 50% medicinal ethanol aqueous solution, and prepare a mint slurry with a concentration of 20%;
[0048] (3) Put the glyceryl monostearate pellet core in the coating granulator, make (2) an atomized slurry through compressed air (pressure 0.2MPa), keep the pellet temperature at 25°C to 30°C, and spray the slurry The ro...
Embodiment 2
[0056] Example 2: batch number 20080202
[0057] Cyproheptadine Hydrochloride 54.5g
[0058] Peppermint Pellets 201g
[0059] Stearic acid 825g
[0060] Glyceryl monostearate 515g
[0061] Triethanolamine 100g
[0062] White Vaseline 720g
[0063] Glycerin 720g
[0064] Ethylparaben 10g
[0065] Fragrance 10ml
[0066] Purified water 7500g
[0067] 1000 pieces
[0068] Prepared according to the method described in Example 1. The loading capacity is 10g / piece.
Embodiment 3
[0070] Cyproheptadine Hydrochloride 53.5g
[0071] Peppermint Pellets 202g
[0072] Stearic acid 825g
[0073] Glyceryl monostearate 515g
[0074] Triethanolamine 100g
[0075] White Vaseline 720g
[0076] Glycerin 720g
[0077] Ethylparaben 10g
[0078] Fragrance 10ml
[0079] Purified water 7500g
[0080] 1000 pieces
[0081] Prepared according to the method described in Example 1. The loading capacity is 10g / piece.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com