Dual inactivated vaccine lymph for ascites disease of cultivated flounder and preparation method
A technology of inactivating bacteria and ascites disease, applied in antibacterial drugs, pharmaceutical formulas, bacterial antigen components, etc., can solve problems such as food safety, instability, and restrictions on the healthy development of the flounder aquaculture industry, so as to improve safety and avoid Harmful effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
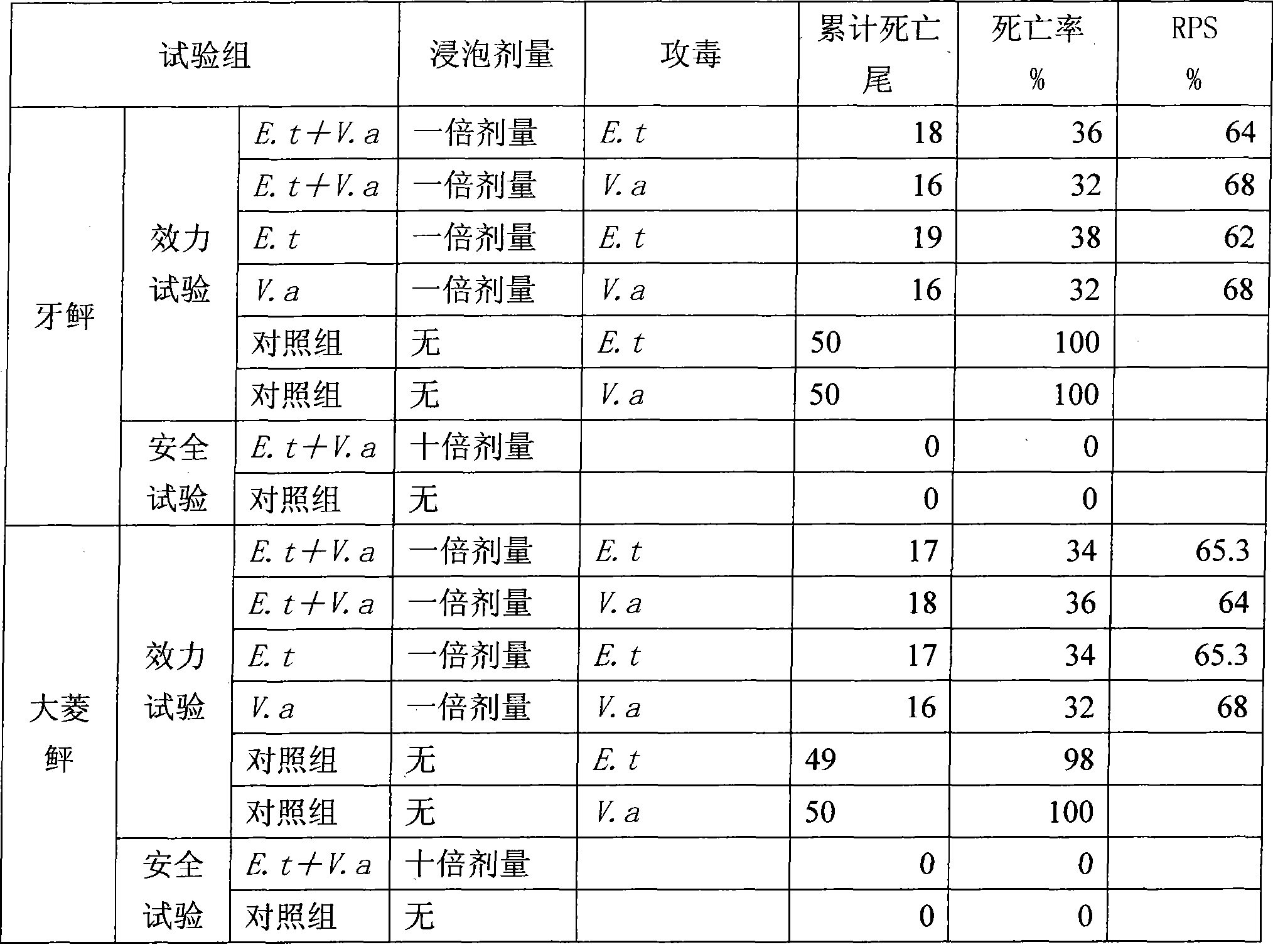
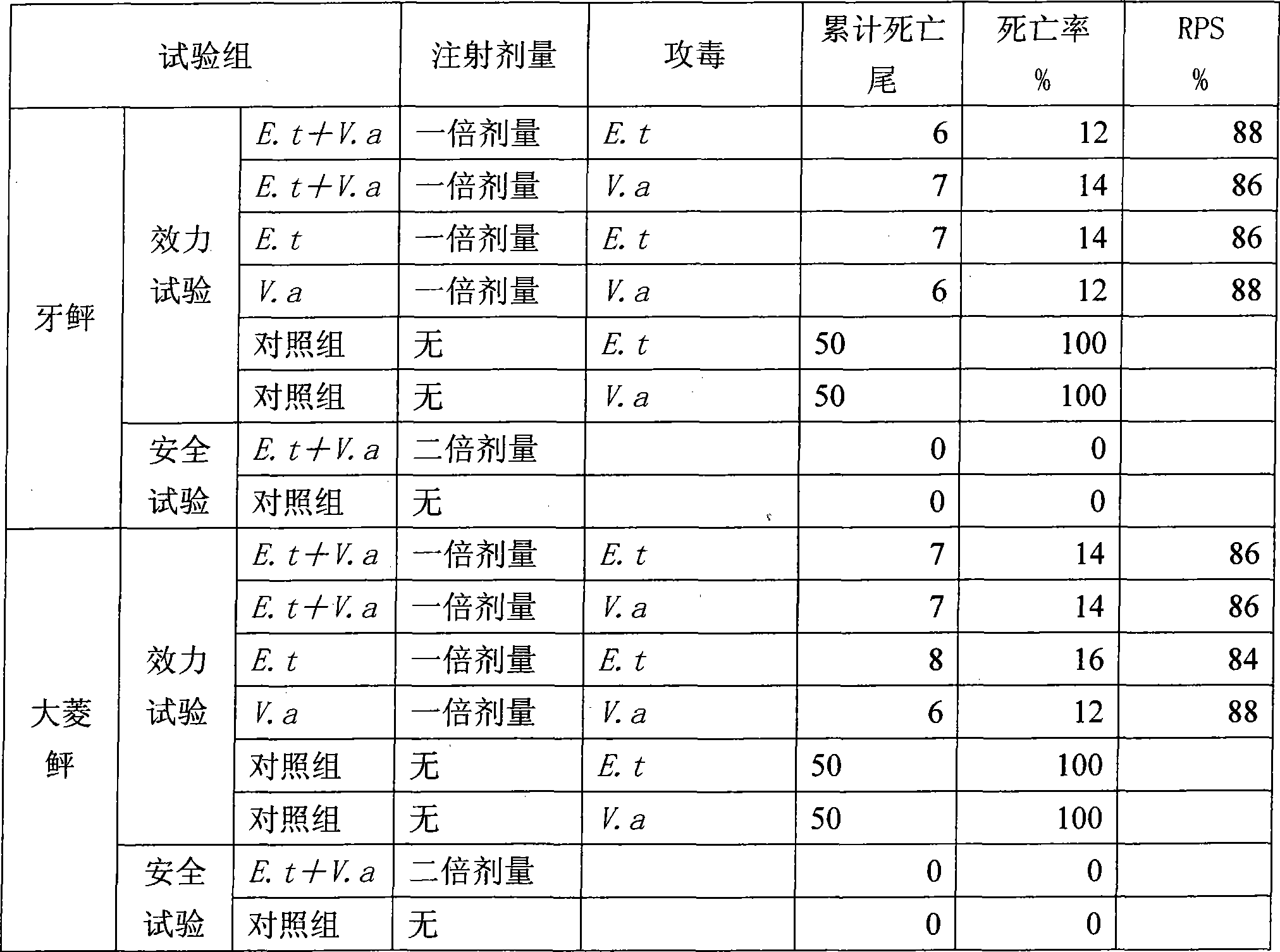
[0024] A dual inactivated bacterium vaccine for cultured flounder ascites disease, the preparation method of which is as follows:
[0025] (1) prepare inactivated bacterial vaccine of Edwardsiella tarda: activate Edwardsiella tarda bacterial strain (ATCC 15947 bacterial strain), inoculate in liquid culture medium (liquid culture medium is that 25gNaCl is added in 1 liter of nutrient broth culture medium to make) cultured culture medium) at 32°C to 37°C in an air bath shaker for 36 hours, terminate the culture when Edwardsiella tarda grows to the plateau stage, harvest, add formaldehyde, so that the final volume percentage concentration of formaldehyde is 0.5%, the temperature The temperature is 35°C, the inactivation time is 48 hours, shake well every 8 hours during this period, carry out the sterility test and formaldehyde residue test according to the "Chinese Veterinary Pharmacopoeia" sterility test and formaldehyde residue test, and the test is qualified, that is, the slow ...
Embodiment 2
[0037] A dual inactivated bacterium vaccine for cultured flounder ascites disease, the preparation method of which is as follows:
[0038] Edwardsiella tarda and Vibrio alginolyticus isolated from the kidney of flounder with ascites disease were purified by single colonies to obtain Edwardsiella tarda and Vibrio alginolyticus strains, which were respectively inoculated on ordinary nutrient agar plates (1 liter of ordinary nutrient agar Add 20g NaCl), culture in a 33°C-35°C bacterial incubator for 24h, wash the bacterial lawn with 0.85% sterile saline, vortex repeatedly to disperse the sticky bacteria, add an appropriate amount of formaldehyde to make the formaldehyde in the bacteria The final volume percent concentration in the liquid is 0.5%, inactivated at room temperature for 48 hours, and shaken every 8 hours. According to the sterility test and formaldehyde residue test method of "Chinese Veterinary Pharmacopoeia", the sterility test and formaldehyde residue test were car...
Embodiment 3
[0049] A double inactivated bacterium vaccine for cultured flounder ascites disease is prepared by the following method:
[0050] (1) Preparation of an inactivated vaccine of Edwardsiella tarda: activate the strain of Edwardsiella tarda, inoculate it in a solid medium, cultivate it at 32° C. to 35° C. for 48 hours, harvest when the Edwardsiella tarda grows to the plateau stage, Inactivate, carry out sterility test, make Edwardia tarda inactivated bacterin vaccine after passing the test, and described solid culture medium is 2216E culture medium;
[0051] (2) Preparation of inactivated Vibrio alginolyticus vaccine: activate the Vibrio alginolyticus strain, inoculate it in a solid medium, culture it at 28°C to 30°C for 48 hours, harvest when the Vibrio alginolyticus grows to the plateau stage, Inactivate, carry out sterility test, promptly make Vibrio alginolyticus inactivated bacterin vaccine after passing the test, and described solid culture medium is 2216E culture medium;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com