Membrane penetrating polypeptide for specific inhibition of hepatitis b virus assembly and replication
A technology of hepatitis B virus and membrane penetration, which is applied in the field of biomedicine, can solve the problems of low membrane penetration, impossibility of practical application, and poor dimensional stability, so as to inhibit replication and secretion, improve antiviral effect, and reduce core Effects at the protein subunit level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 Preparation of recombinant protein of the present invention
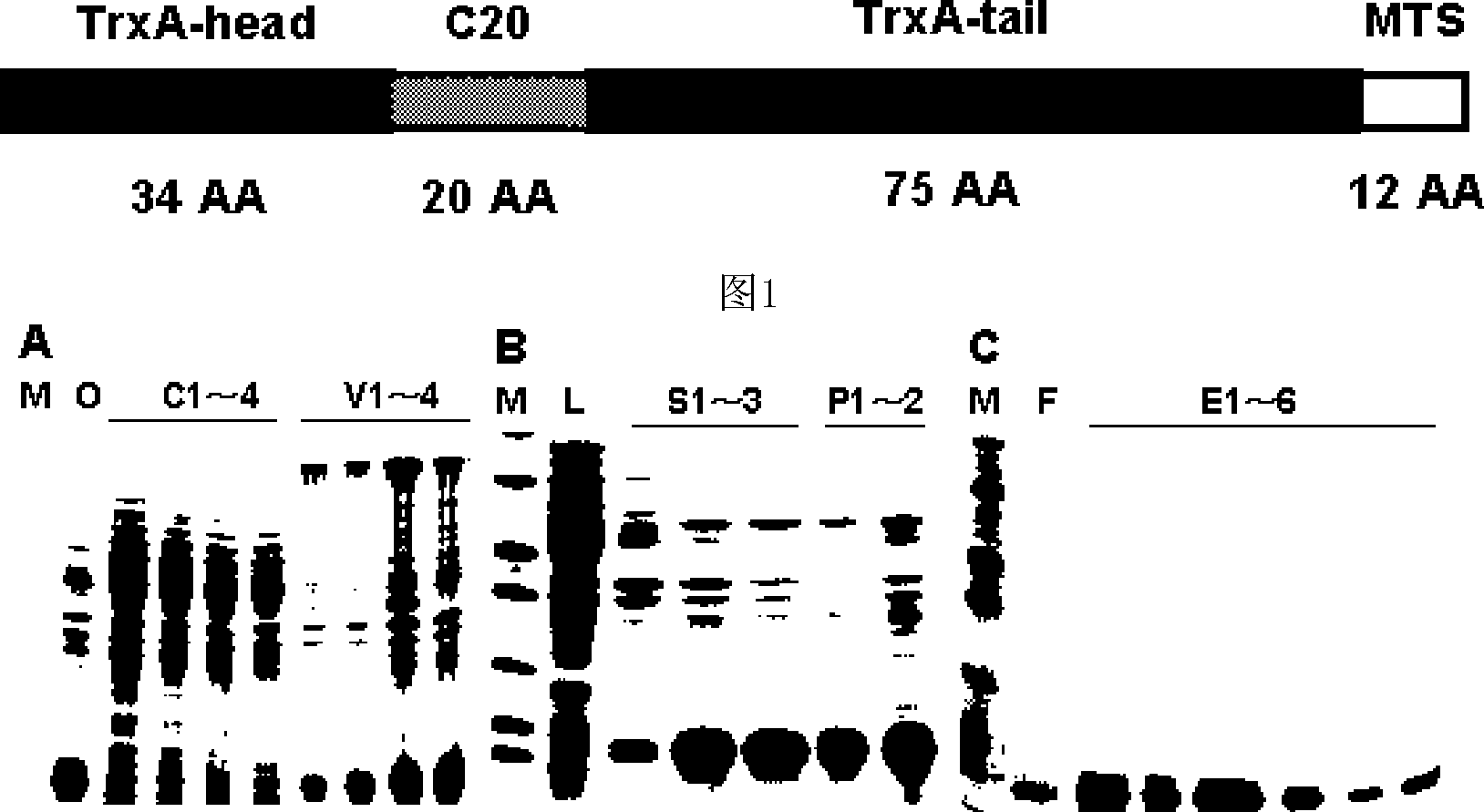
[0055] According to the characteristics of HBV virus, the membrane-penetrating polypeptide drug C20-MTS, which specifically blocks the assembly and replication of hepatitis B virus, is designed with HBV core protein as the target site.
[0056] Firstly, screening of polypeptide sequences that can specifically and strongly bind to HBV core protein is used as the basis for drug development;
[0057] According to literature reports, and using the humanized peptide aptamer screening system, a polypeptide sequence consisting of 20 AAs with the strongest binding ability to HBV core protein was identified, named C20, and its amino acid sequence was:
[0058] SFYSVLFLWGTCGGFSHSWY.
[0059] In order to meet the requirements of clinical application, the sequence (C20) is molecularly modified:
[0060] 1. Select the humanized backbone protein TrxA to reduce the immunogenicity of heterologous proteins; ins...
Embodiment 2
[0077] Embodiment two in vitro pharmacodynamic studies
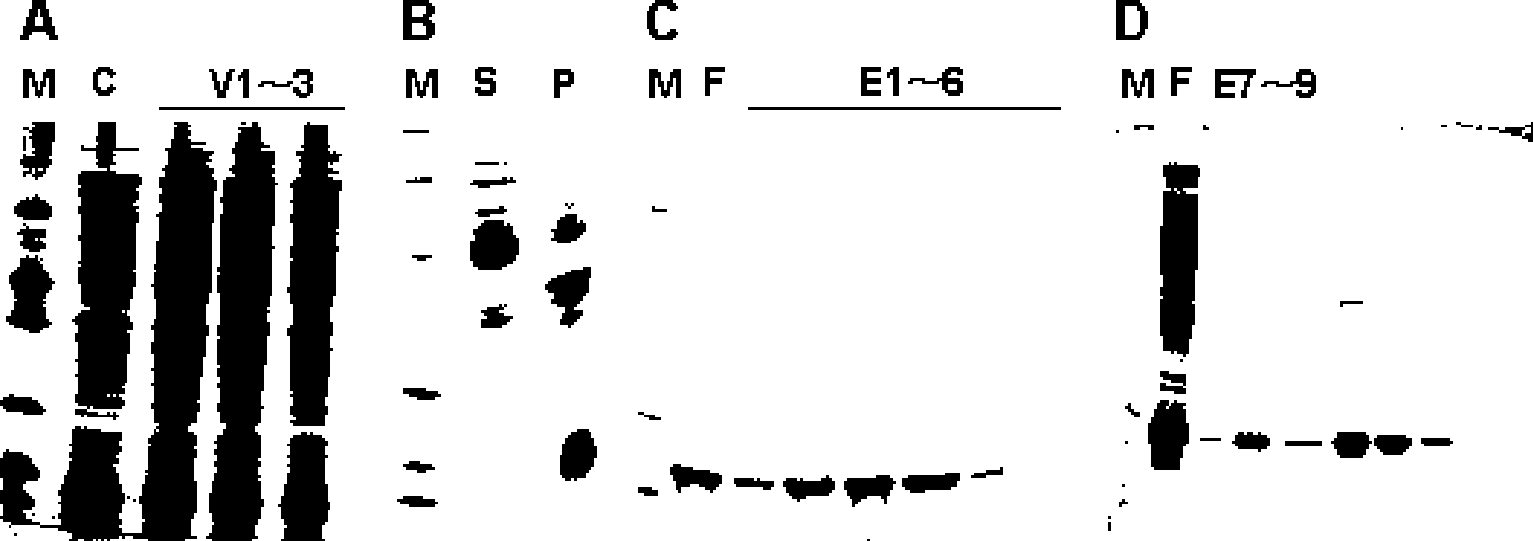
[0078] The HBV transgenic HepG2.215 cell line was obtained to verify the efficacy of the in vitro model, and C20 was used as a control (without adding MTS sequence) to test the membrane penetration ability of C20-MTS.
[0079] ELISA can detect the content of HBsAg in the supernatant of cell culture medium, the level of HBsAg antigen on the surface of cell culture medium and plasma, which can indicate the content of secreted virus particles.
[0080] Western blot detection of HBV core protein subunit content in cell lysate; determination of intracellular HBV core protein subunit DNA content to indicate the formation of HBV core protein subunit
[0081] The probe method was used to detect the HBV DNA content in the cell lysate and determine the intracellular HBV DNA content to indicate the replication of viral DNA.
[0082] The results are as follows: In the in vitro experiment, C20-MTS (polypeptide drug with MTS sequence...
Embodiment 3
[0085] Embodiment three in vivo pharmacodynamics research
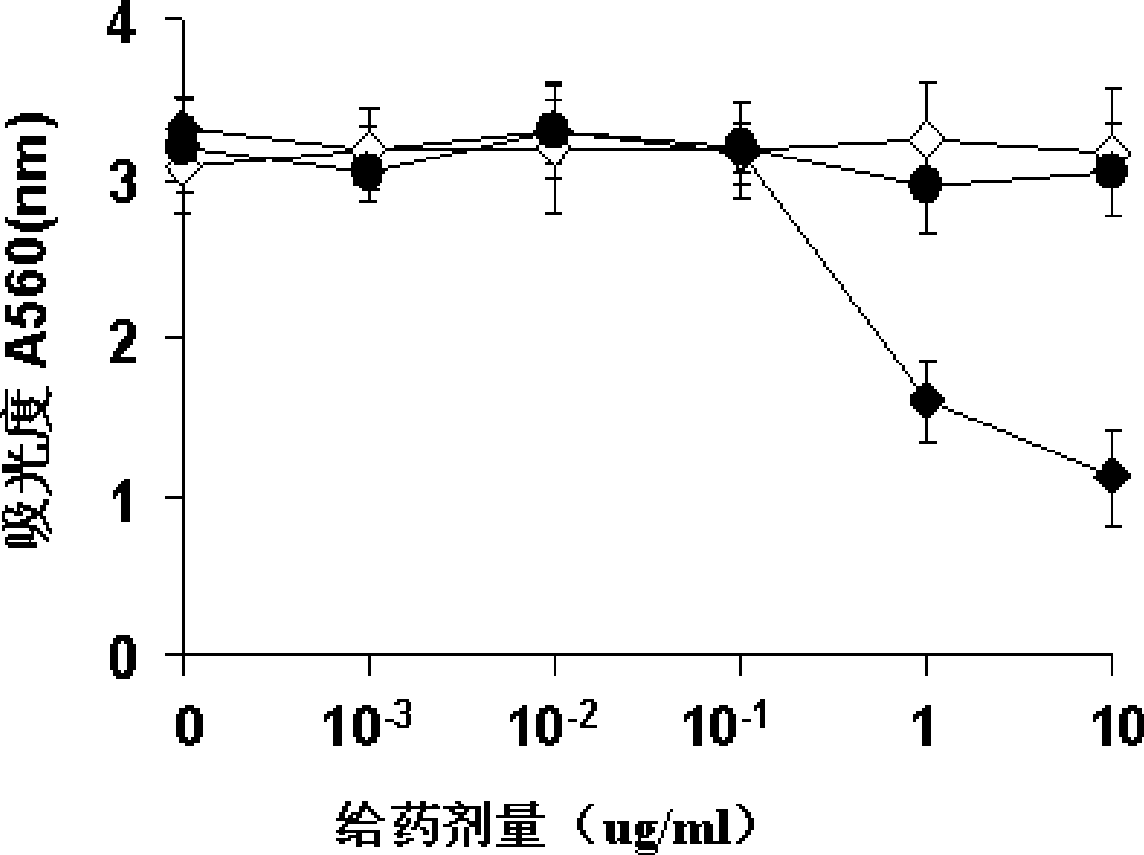
[0086] Use the HBV transgenic Balb / c mouse model (mouse source: Guangzhou Military Region Hospital.) to detect the in vivo efficacy of the protein of the present invention, and use C20 as a control (without adding MTS sequence) to test the membrane penetration ability of C20-MTS.
[0087] The HBsAg content in peripheral blood of mice was detected by ELISA.
[0088] The content of HBV core protein subunit in mouse hepatocytes was detected by Western blot.
[0089] Detection of HBV DNA content in mouse hepatocytes by probe method.
[0090] In vivo experiments, we use the HBV transgenic mouse model for drug efficacy verification. According to the results of in vitro experiments, we chose to administer once every 2 days. The results showed that: when the dose was 2.5 mg / kg or more, the level of HBsAg secretion could be reduced; when the dose was 5 mg / kg, the level of HBsAg was detected on the 8th day Reduced by about 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com