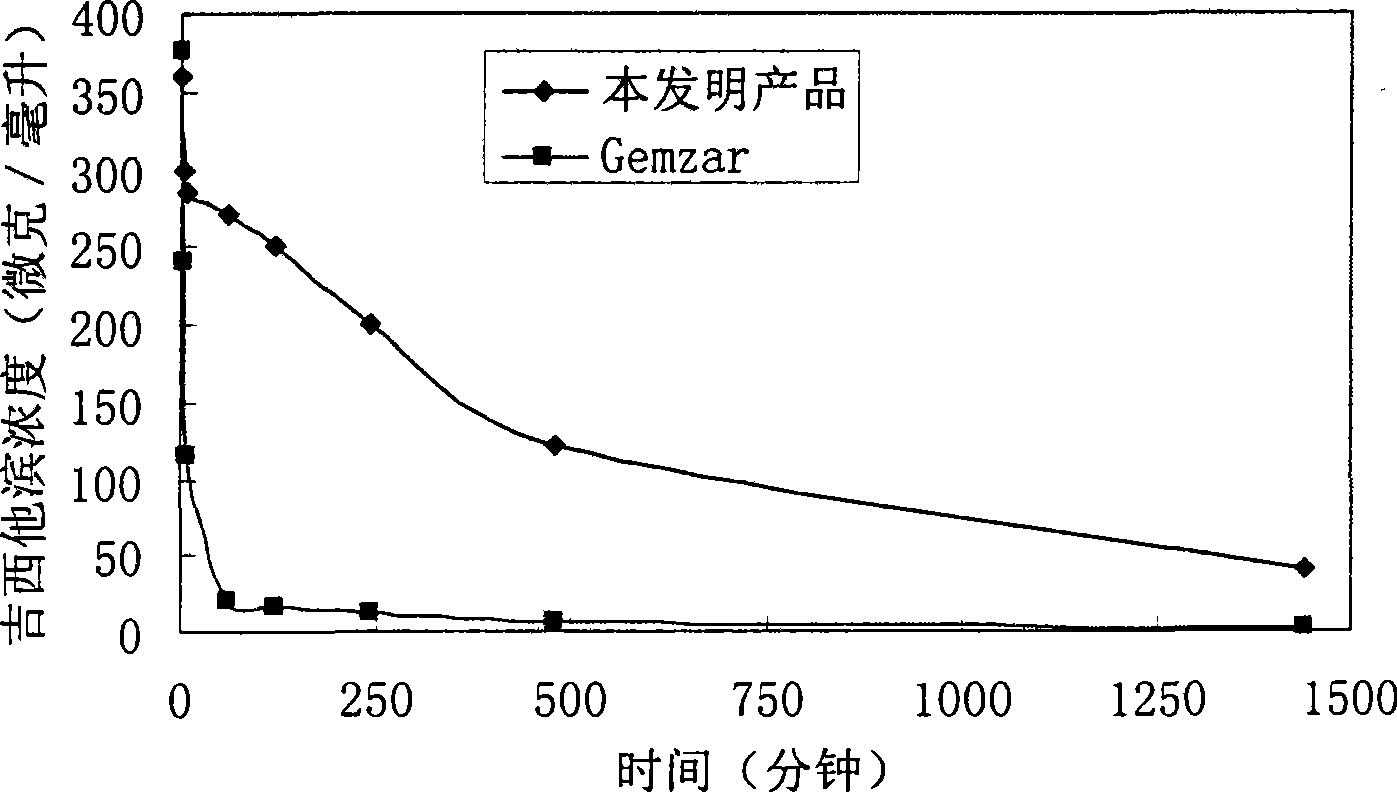
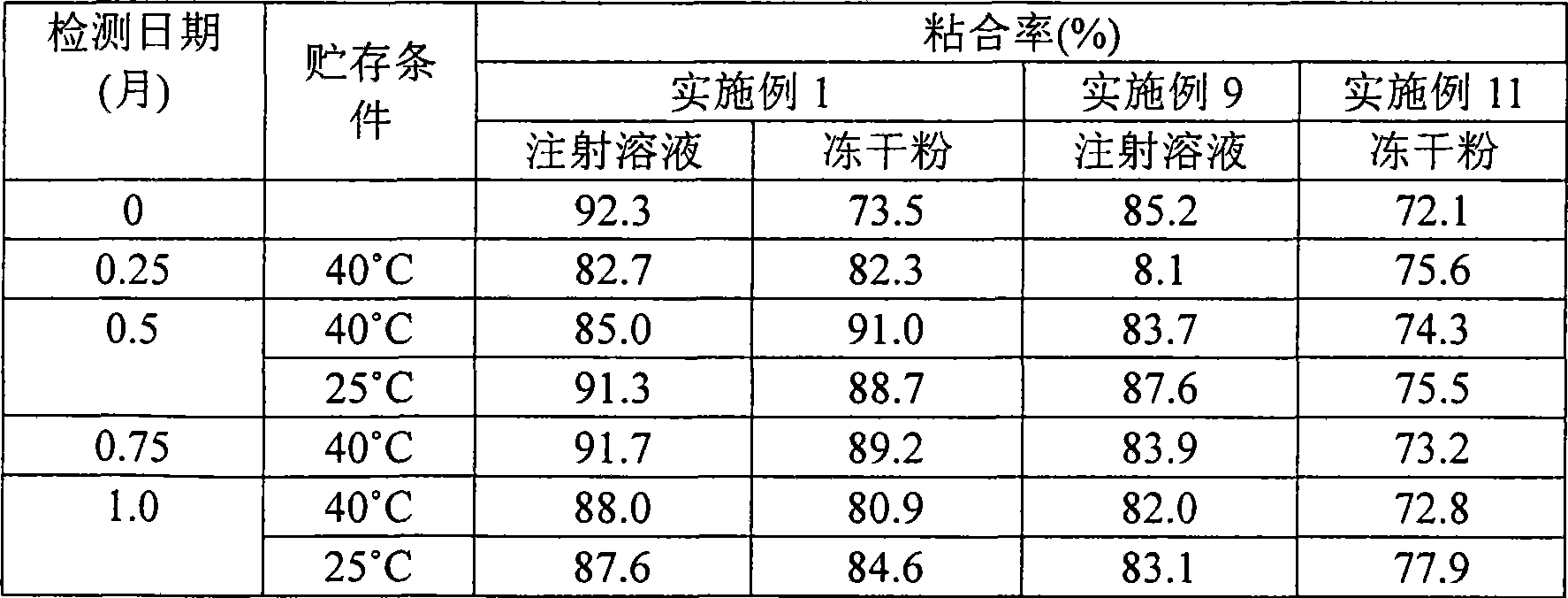
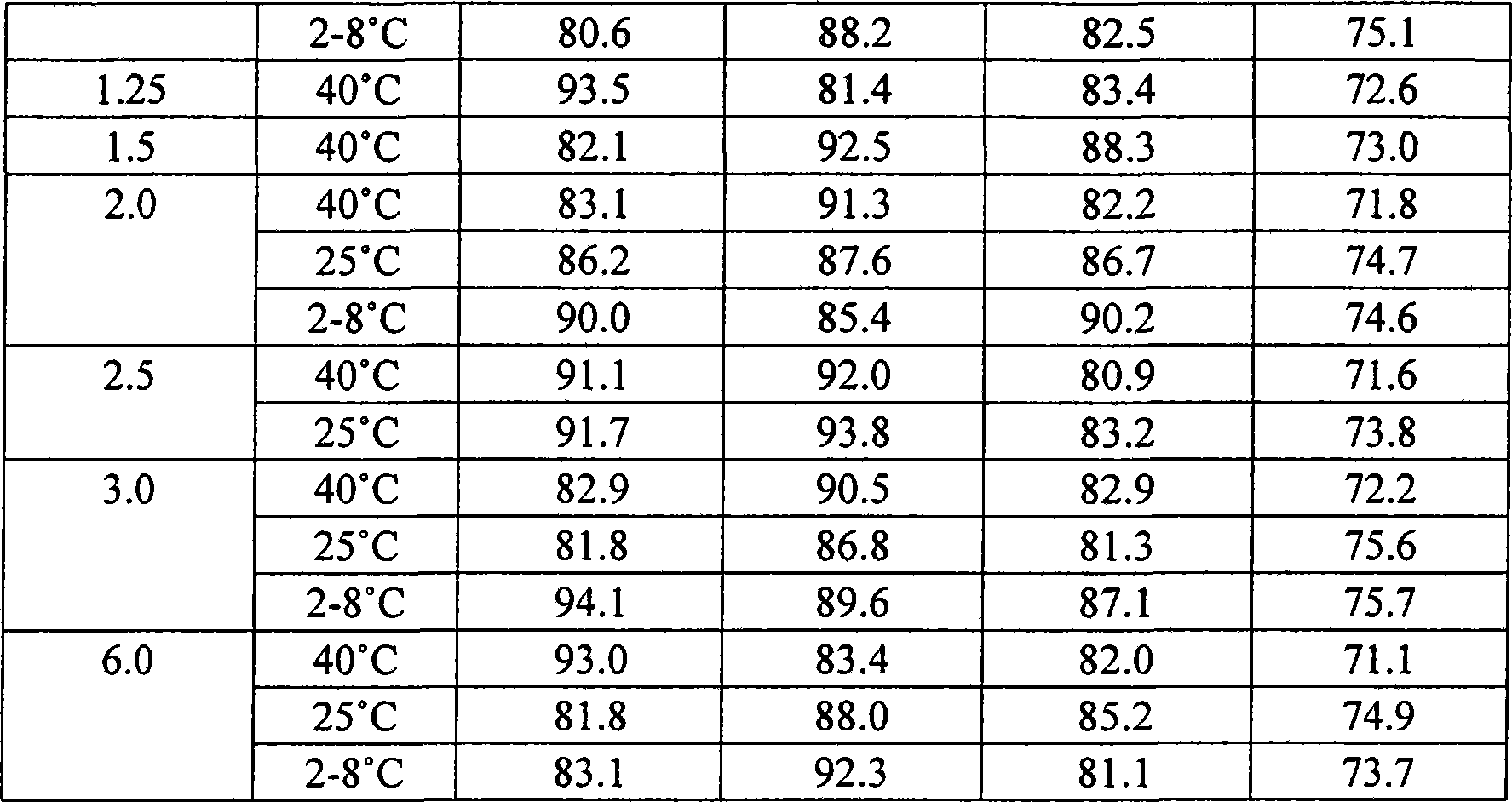
Gemcitabine hydrochloride or gemcitabine composition
A technology of gemcitabine hydrochloride and gemcitabine, which is applied in the directions of drug combination, non-active components of polymer compounds, drug delivery, etc., can solve the problems of reduced stability, clinical efficacy, small molecular weight, etc., so as to improve drug tolerance and improve stability , Simple and convenient clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0034] Preparation Example 1: 100ml gemcitabine hydrochloride solution and 100mg / bottle gemcitabine hydrochloride lyophilized powder
[0035] Preparation Composition: Gemcitabine Hydrochloride 1000mg
[0036] Human serum albumin (~MW66K) 10000mg
[0037] Polyvinylpyrrolidone (K17) 10000mg
[0038] Disodium hydrogen phosphate 1420mg
[0039] Mannitol 3000mg
[0040]Vitamin C 0.5mg
[0041] appropriate amount of water for injection
[0042] Hydrochloric acid or sodium hydroxide appropriate amount
[0043] Preparation method: Dissolve disodium hydrogen phosphate in water for injection, adjust the pH value to 7.5 and prepare disodium hydrogen phosphate buffer solution. Dissolve human serum albumin, polyvinylpyrrolidone, mannitol, and vitamin C in disodium hydrogen phosphate buffer solution, add gemcitabine hydrochloride after complete dissolution, adjust the pH value to 7.5 after complete dissolution, and dilute to ...
preparation Embodiment 2
[0044] Preparation Example 2: 10ml gemcitabine hydrochloride solution
[0045] Preparation Composition: Gemcitabine Hydrochloride 150mg
[0046] Bovine serum albumin (~MW69K) 600mg
[0047] Polyvinylpyrrolidone (K17) 1000mg
[0048] Sodium dihydrogen phosphate 120mg
[0049] Sucrose 300mg
[0050] EDTA0.05mg
[0051] appropriate amount of water for injection
[0052] Hydrochloric acid or sodium hydroxide appropriate amount
[0053] Preparation method: Dissolve sodium dihydrogen phosphate in water for injection, adjust the pH value to 7.4, and prepare sodium dihydrogen phosphate buffer solution. Dissolve bovine serum albumin, polyvinylpyrrolidone, sucrose and EDTA in sodium dihydrogen phosphate buffer solution, add gemcitabine hydrochloride after complete dissolution, adjust the pH value to 7.4 after complete dissolution, dilute to volume with the prepared buffer solution, and filter to sterilize Finally, the solution is filled in type ...
preparation Embodiment 3
[0054] Preparation Example 3: 10ml gemcitabine hydrochloride solution
[0055] Preparation Composition: Gemcitabine Hydrochloride 50mg
[0056] Human serum albumin (~MW66K) 1000mg
[0057] Polyvinylpyrrolidone (K17) 500mg
[0058] Citric acid 154mg
[0060] Vitamin C 0.05mg
[0061] Mannitol 100mg
[0062] appropriate amount of water for injection
[0063] Hydrochloric acid or sodium hydroxide appropriate amount
[0064] Preparation method: Dissolve citric acid in water for injection, adjust the pH value to 7.0, and prepare a citric acid buffer solution. Dissolve human serum albumin, polyvinylpyrrolidone, sodium chloride, vitamin C and mannitol in citric acid buffer solution, add gemcitabine hydrochloride after complete dissolution, adjust the pH value to 7.0 after complete dissolution and use the prepared buffer solution to volume After filter sterilization, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com