Pyrethroid pesticide artificial antigen, antibody and preparation thereof
A pyrethroid, artificial antigen technology, applied in chemical instruments and methods, animal/human proteins, serum albumin and other directions, can solve the problems of high preparation cost, many synthesis steps, low sensitivity of antibody analysis, etc., and achieve high sensitivity , The effect of low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) The hapten synthesis method is as follows:
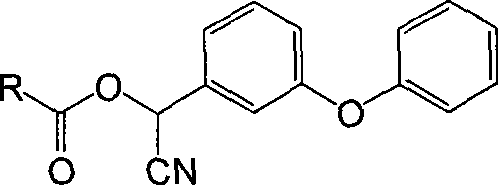
[0032] Dissolve 1.63g (5mmol) of α-cyano m-phenoxybenzyl alcohol in 5mL of dichloromethane to form a solution with a concentration of 1mol / L, and add it to the reaction flask after being cooled to the reaction temperature; 0.62g (6mmol ) succinic anhydride was dissolved in 2mL of dichloromethane to form a solution with a concentration of 3mol / L, and after being cooled to the reaction temperature, it was added dropwise to the reaction flask. After the dropwise addition, the mixture was stirred and reacted for 12 hours, and the reaction temperature was 14°C. The molar ratio of the reaction raw materials α-cyano-m-phenoxybenzyl alcohol to succinic anhydride is 1:1.2. After the reaction, pyrethroid haptens are generated (where R=CN).
[0033] The structure of the generated type I pyrethroid hapten was identified by electrospray mass spectrometry and nuclear magnetic resonance, and the identification result was: ESI-MS: 324 (...
Embodiment 2
[0046] 1) Synthesize the hapten according to the method in the invention patent "Type I pyrethroid pesticide general hapten and its synthesis method" (application number: ) that the applicant has applied for, and the synthesis method is as follows:
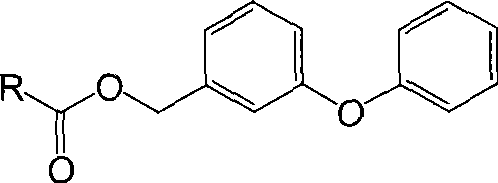
[0047] Dissolve 1.18g (5mmol) of m-phenoxybenzyl alcohol in 5mL of dichloromethane to make a 1mol / L solution, cool to 18°C, and add to the reaction flask after cooling to 18°C; add 1.02g of succinic anhydride ( 10mmol) was dissolved in 10mL of dichloromethane to make a 1mol / L solution, cooled to 18°C and then added dropwise to the reaction flask, and stirred for 15 hours after the dropwise addition, the reaction temperature was 18°C, The molar ratio of benzyl alcohol and succinic anhydride is 1:2. After the reaction, the method of rotary evaporation was used to remove most of the organic solvents, and then the method of silica gel column chromatography was used to purify to obtain 1.47g (4.9mmol) of white solid product, and the ...
Embodiment 3
[0061] 1) Pyrethroid hapten (where R=CN) was synthesized and identified as in step 1) of Example 1.
[0062] 2) Coupling the synthesized hapten compound with protein to prepare artificial antigen, including immune antigen and coating antigen, the method is as follows:
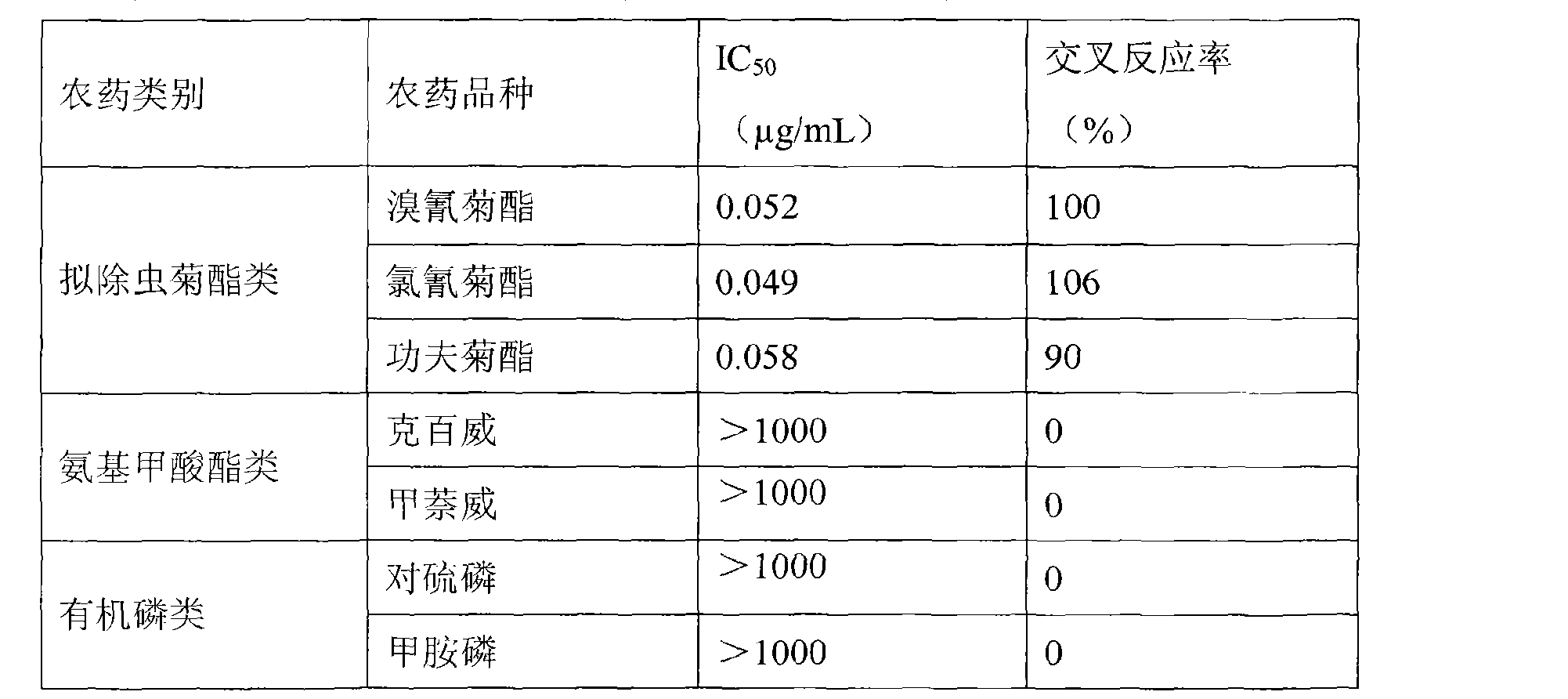
[0063] The hapten synthesized above 0.11g (0.32mmol) (wherein R=CN), equimolar N, N-dicyclohexylcarbodiimide and equimolar N-hydroxysuccinimide were dissolved in 0.6mL of N, N-dimethylformamide, Stir the reaction at 20°C for 4 hours. After the reaction is over, place the reaction mixture in an environment of 4°C overnight (more than 10 hours), then centrifuge, take 0.48mL of the supernatant, divide it into two equal parts, and slowly add it to In 3mL of carbonate buffer solution with a concentration of 15mg / mL hemocyanin, react under stirring for 14 hours. After the reaction is completed, put it into a dialysis bag, and dialyze 8 times with conventional phosphate buffer solution of pH 7.4 as dialysate , wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com