Method for preparing polymer aqueous solution formulation of taxane anti-tumor medicament
A technology of anti-tumor drugs and taxanes, which is applied in the directions of anti-tumor drugs, drug combinations, and pharmaceutical formulations, can solve the problems of ethanol irritation of surfactants, prone to allergic reactions, and harsh storage conditions, and achieves improved compliance. It is beneficial to the distribution and metabolism, and the effect of convenient medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
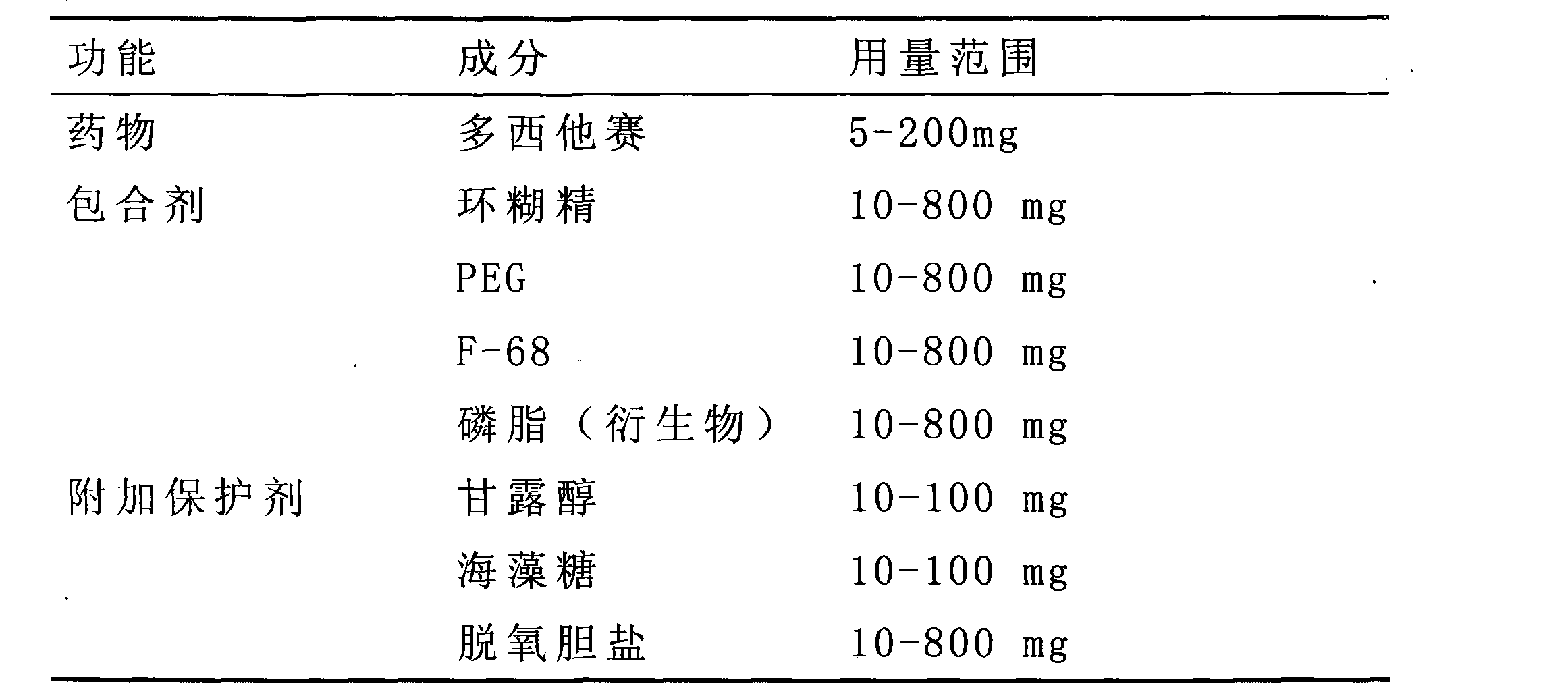
[0051] Raw and auxiliary materials used in the reagent: paclitaxel 50mg, soybean lecithin 1.5g, sodium deoxycholate 1.8g, absolute ethanol 30ml, trehalose 80mg. Phosphate buffer saline (PBS, pH=7.4) 10ml.
[0052] Description of the preparation process: 1. Weigh paclitaxel, soybean lecithin, and sodium deoxycholate and dissolve them in absolute ethanol. 2. Rotate evaporation at 40°C to remove all ethanol, so that drugs, phospholipids, etc. form a dry light yellow film on the bottom of the bottle. 3. Add the PBS solution dissolved with trehalose, and dissolve the film with ultrasound or vibration. A light yellow homogeneous transparent solution was obtained. 4. Dialyze overnight with a semi-permeable membrane with a permeability limit of 8000, and replace the external fluid at intervals. A colorless homogeneous transparent solution was obtained. 5. Filter with a 0.22 μm microporous membrane. 6. Subpackage, label after freeze-drying, and store at room temperature.
[0053]...
Embodiment 2
[0055] Raw and auxiliary materials used in the reagent: 30 mg of docetaxel, 1.90 g of soybean lecithin, 1.40 g of sodium deoxycholate, PBS (pH7.2), 0.40 g of trehalose.
[0056] Description of the preparation process: the preparation process is the same as in Example 1.
[0057] Preparation traits: colorless, transparent and uniform solution, which can be stored for about 60 days. The shape of the freeze-dried product did not change significantly.
[0058] Diluted with 5% glucose solution and 0.9% normal saline respectively according to the concentration of 100mg drug / 500ml, 150mg drug / 500ml, 200mg drug / 500ml, it can exist stably for more than 3 months.
Embodiment 3
[0060] Raw and auxiliary materials used in the reagent: 30 mg of docetaxel, 0.61 g of hydroxypropyl-β-cyclodextrin, 80 mg of PEG-8000, 40 mg of F-6840 mg, 40 mg of sorbitol, and an appropriate amount of ethanol.
[0061] Description of the preparation process: 1. Accurately weigh docetaxel and F-68, dissolve in absolute ethanol, and freeze to aid in dissolution. A homogeneous colorless and transparent solution was prepared. 2 Accurately weigh hydroxypropyl-β-cyclodextrin, PEG, and sorbitol, and dissolve them in water for injection to obtain a uniform colorless and transparent solution. Slowly transfer the ethanol solution of the drug into the polymer solution in 3 portions. Avoid the appearance of air bubbles, vortices and white flocs. Mixed homogeneous colorless transparent solution. 4 Filter with a 0.22 μm microporous membrane. Pack in 5 aliquots, label after freeze-drying, and store at room temperature.
[0062] Preparation traits: colorless, transparent and uniform so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com