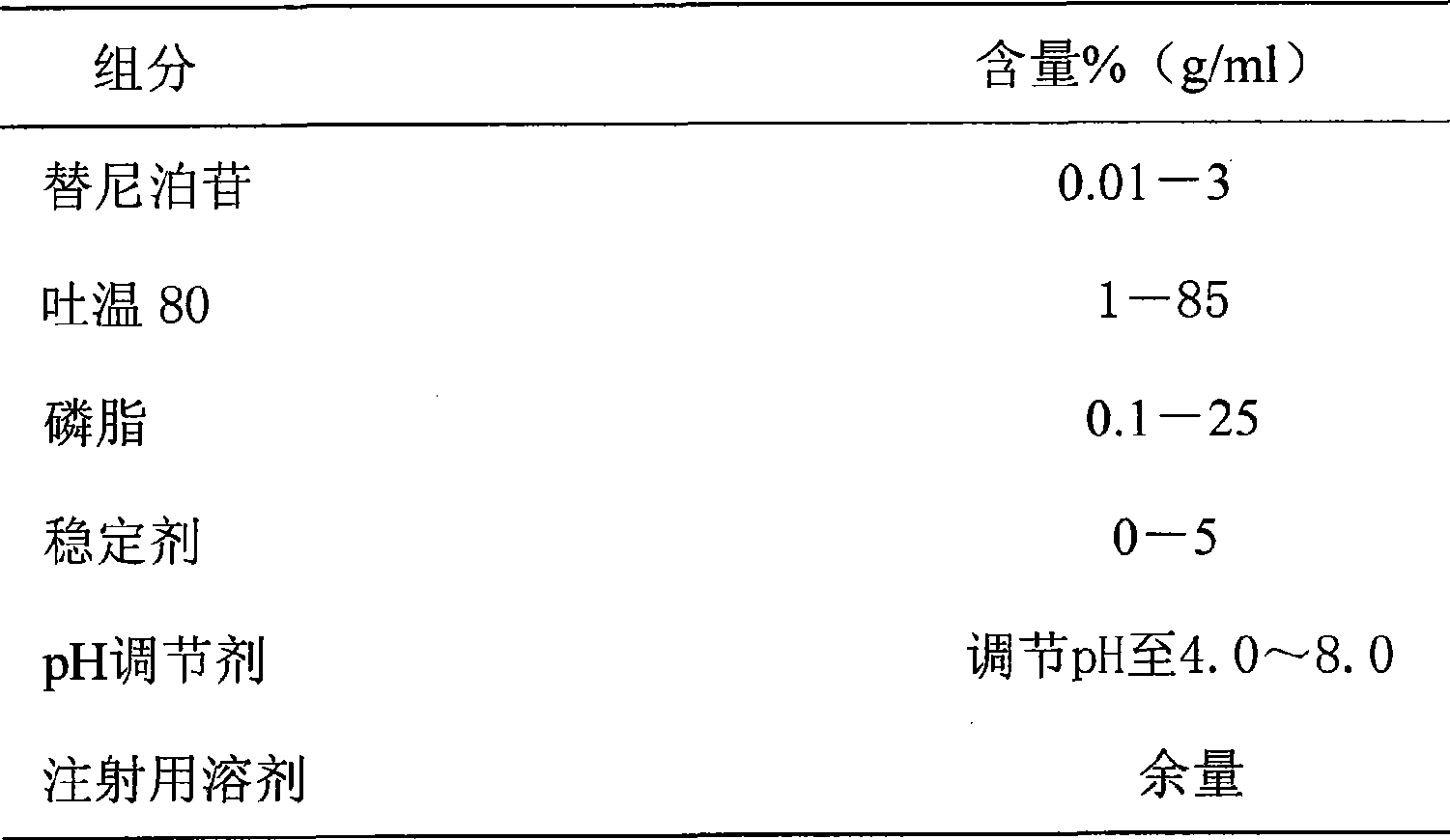
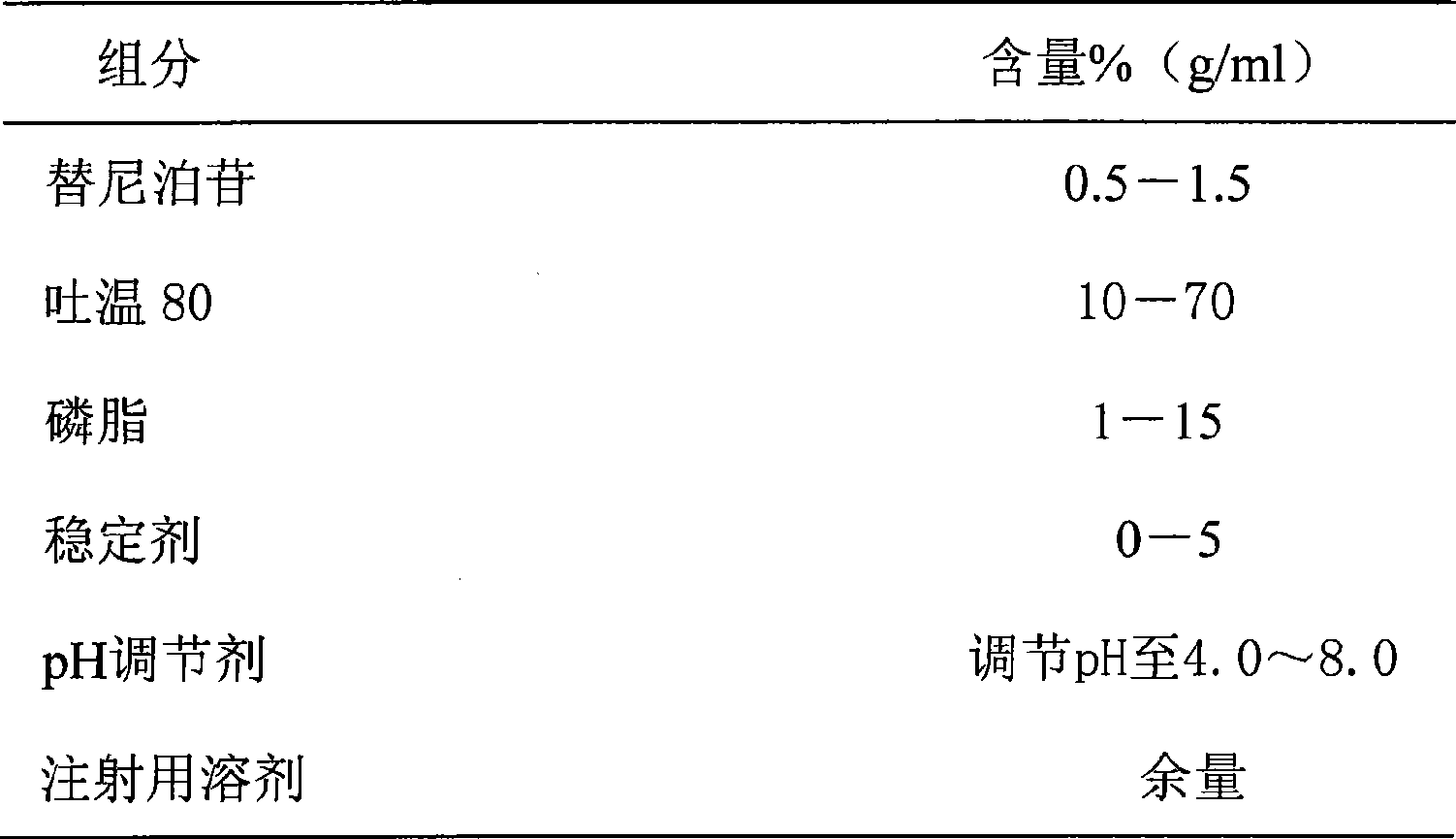
Teniposide injection and preparation method thereof
A technology of niposide injection and teniposide, which is applied in the field of medicine, can solve problems such as drug leakage, thermodynamic instability, and particle size increase, achieve good stability and water solubility, reduce work burden, and improve compliance sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of Teniposide Injection
[0019] Take 1 g of teniposide, add 3 ml of benzyl alcohol, 6 ml of N,N dimethylacetamide, stir and dissolve at 60°C as solution 1; take 15 ml of absolute ethanol, add 45 g of Tween-80, inject Use 15 grams of soybean lecithin and 1 gram of cholesterol, stir and dissolve at 60°C as solution 2; mix solution 1 and solution 2 evenly, add absolute ethanol to 100 ml, adjust the pH to 5.0 with fumaric acid, and then add 0.3 grams of Use activated carbon for needles, absorb at 30°C for 30 minutes, filter through a 0.45 μm microporous membrane, and then filter and sterilize with a 0.22 μm microporous membrane, then dispense it.
Embodiment 2
[0020] Example 2 Preparation of Teniposide Injection
[0021] Take 0.1 g of teniposide, add 1 ml of benzyl alcohol and 5 ml of absolute ethanol, stir and dissolve at 40°C as solution 1; take 35 ml of absolute ethanol, 10 ml of glycerin, and 15 ml of polyethylene glycol 200 and mix well. Add 3 grams of Tween-80, 25 grams of soybean lecithin for injection, and 0.5 grams of glycerol monooleate, stir and dissolve at 40°C as solution 2; mix solution 1 and solution 2 evenly, add absolute ethanol to 100 ml, and use Adjust the pH to 4.0 with acetic acid, then add 0.05 g of activated carbon for needles, absorb at 60°C for 100 minutes, filter with a sand filter stick, sub-package, and sterilize by high-pressure steam at 105°C for 45 minutes.
Embodiment 3
[0022] Example 3 Preparation of Teniposide Injection
[0023] Take 0.01 g of teniposide, add 5 ml of propylene glycol, and dissolve it with conventional ultrasound as solution 1; take 70 ml of propylene glycol, 10 ml of polyethylene glycol 300 and mix well, add 1 g of Tween-80, and 0.1 g of egg yolk phospholipid for injection 1. Poloxamer 188 (F68) 5 grams, according to conventional ultrasonic dissolution as solution 2; Mix solution 1 and solution 2 evenly, add propylene glycol to 100 ml, adjust pH to 6.5 with citric acid and sodium hydroxide, then add 0.01 gram of needles with activated carbon, adsorbed at 80°C for 25 minutes, filtered with a vertical melting funnel, packed separately, and sterilized by high-pressure steam at 121°C for 20 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com