Nicarbazin and ethopabate nano suspension agent and preparation method thereof
A technology of ethoxybenzamide and nanosuspension is applied in the directions of amide active ingredients, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve the problem of inconvenient, inaccurate and wasteful administration of drugs. and other problems, to achieve the effect of solving the inconvenience of administration, prolonging the residence time, and improving the bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Components:
[0036] Component Dosage
[0037] Nicarbazine 25% (W / V)
[0038] Ethoxylate 1.6% (V / V)
[0039] Ethanol 3% (V / V)
[0040] Sodium Lauryl Sulfate 0.06% (W / V)
[0041] Glycerin 10% (V / V)
[0042] Sodium Citrate 2% (W / V)
[0043] Sodium metabisulfite 0.2% (W / V)
[0044] Methylparaben 0.03% (W / V)
[0045] Purified water 87% (V / V)
[0046] Configuration method:
[0047] (1) Dissolve sodium citrate and sodium metabisulfite in 27ml of purified water, then add nicarbazine, ethopabate, ethanol and sodium lauryl sulfate, and stir to obtain mixture A.
[0048] (2) Take 60% of purified water, add methylparaben, heat to dissolve, place it at room temperature, then add glycerin, and stir to obtain mixture B.
[0049] (3) Pour solution B into solution A, stir and mix well, and then use purified water to fix the solution to 100% (V / V) to obtain mixture C.
[0050] (4) Mixture C is homogenized by a high-pressure homogenizer under a pressure of 100Mpa, and after ho...
Embodiment 2
[0052] Components:
[0053] Component Dosage
[0054] Nicarbazine 12.5% (W / V)
[0055] Ethoxylate 0.8% (W / V)
[0056] Ethanol 2.3% (V / V)
[0057] Sodium Lauryl Sulfate 0.08% (W / V)
[0058] Sodium Carboxymethyl Cellulose 5% (W / V)
[0059] Glycerin 12% (V / V)
[0060] Sodium Citrate 2.5% (W / V)
[0061] Anhydrous sodium sulfite 0.2% (W / V)
[0062] Propylparaben 0.03% (W / V)
[0063] Purified water 86% (V / V)
[0064] Configuration method:
[0065] (1) Dissolve sodium hydrogen citrate into 26ml of purified water, then add nicarbazine, ethopabate, ethanol and sodium lauryl sulfate, and stir to obtain mixture A.
[0066] (2) Take 60ml of purified water, add anhydrous sodium sulfite and propylparaben, heat to dissolve and place it at room temperature, then add sodium carboxymethylcellulose and glycerin, and stir to obtain mixture B.
[0067] (3) Pour mixture B into mixture A and stir to obtain mixture C.
[0068] (4) The mixture C is homogenized by a high-pressure homogenize...
Embodiment 3
[0070] Clinical Efficacy Trial
[0071] 1. Materials
[0072] Premix: nicarbazine, ethopabate premix, purchased from market products.
[0073] Embodiment 1: nicarbazine, ethopabate nano dry suspension.
[0074] 2. Method
[0075] Mix 500 g of the premix into 1000 kg of feed, feed the chickens suffering from coccidiosis at the age of 20 days, and use it continuously for 5 days. Add 500ml of the suspension into 500L of water, mix well, and provide drinking water for 20-day-old chickens suffering from coccidiosis for 5 consecutive days.
[0076] 3. Experimental results
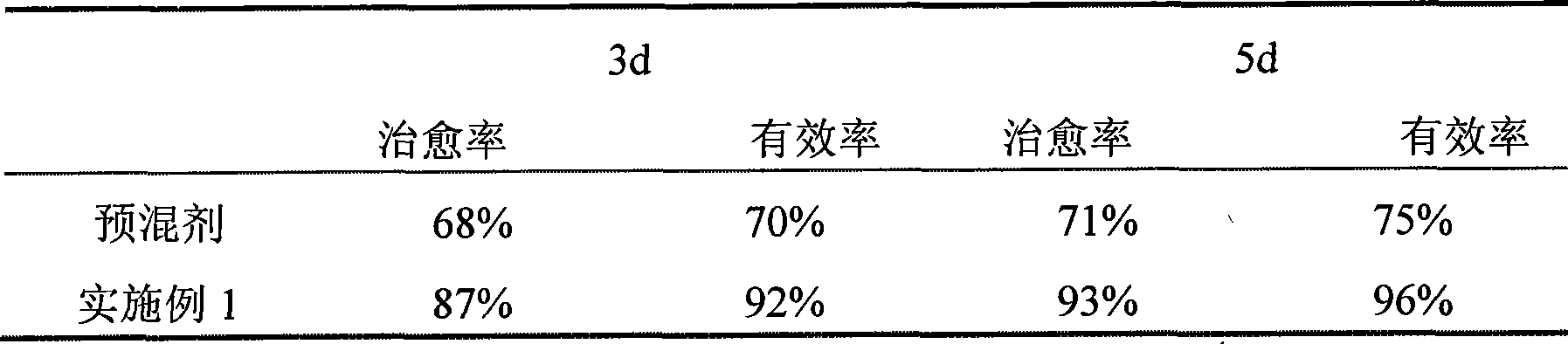
[0077] The therapeutic effect of table 1 premix and embodiment 1 on chicken coccidiosis
[0078]
[0079] As can be seen from the experimental results, compared with the premix, the treatment effect of Example 1 is obviously better than that of the premix, and it is convenient to use and does not waste.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com