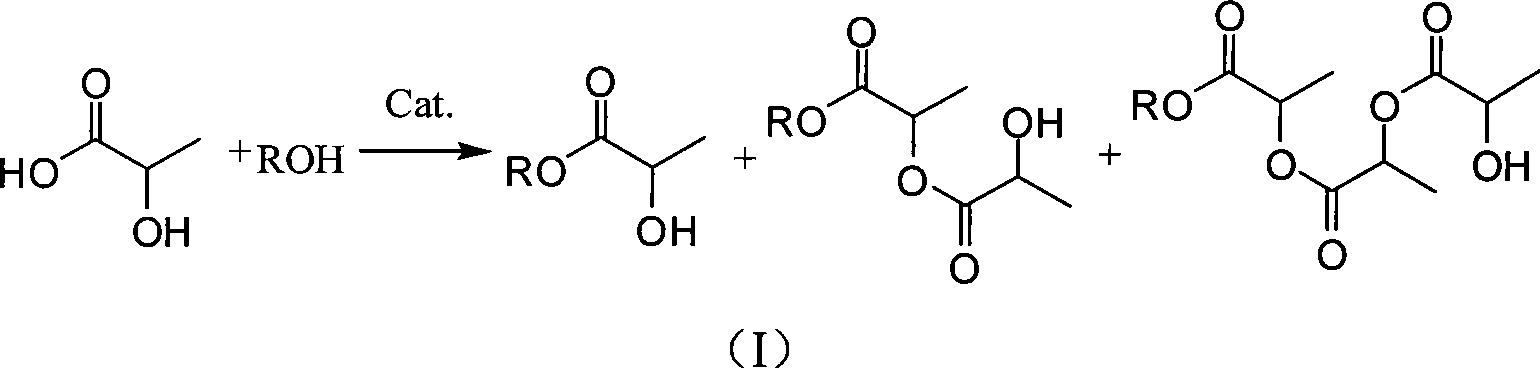
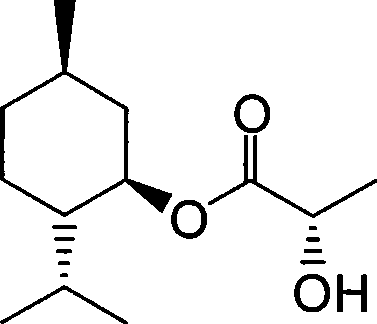
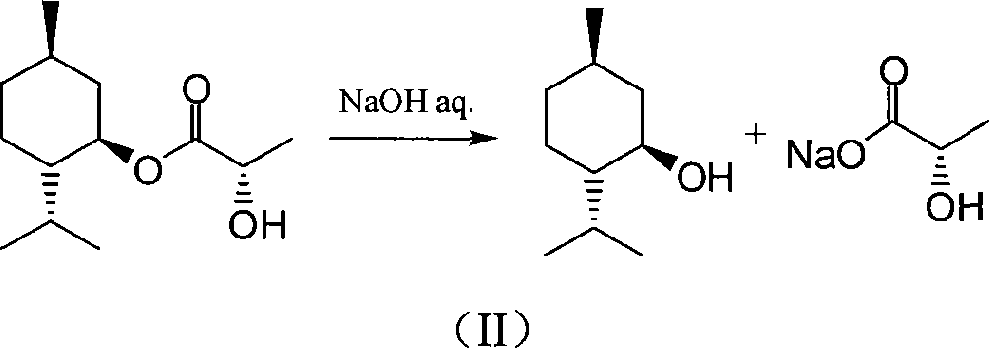
Purification method of lactic acid ester
A purification method and technology of lactic acid ester, applied in the direction of organic chemistry, reaction preparation of ester group and hydroxyl group, etc., can solve the problem of simultaneous hydrolysis of lactic acid ester, and achieve the effects of reducing production costs, simple operation, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Prepare and purify menthol lactate by the method of the present invention, comprise the following steps:
[0035] (1) Preparation of menthol lactate, dimerization and polymer mixture:
[0036] Put quantitative 50g menthol, 50g lactic acid (85%wt) and 50mL toluene respectively into the four-neck flask, slowly drop 0.35g concentrated sulfuric acid in the stirring state, heat and reflux in the oil bath for 2h, collect 15mL water in the water separator, and detect by GC Menthol content <8%wt, the reactant was cooled to room temperature, washed with 10mL of saturated saline, 10% aqueous sodium hydroxide solution to adjust the pH value to 6.5-7.5, and washed again with 10mL of saturated saline, and the solvent was recovered. It is a mixture of menthol lactate, dimer and polymer.
[0037] (2) Degradation of polymers:
[0038] At room temperature, dissolve the mixture obtained in the above steps in 70 mL of anhydrous methanol, add 0.6 g of solid potassium carbonate, stir for ...
Embodiment 2
[0040] Prepare and purify cyclohexanol lactate by the method of the present invention, comprise the following steps:
[0041] (1) Preparation of cyclohexanol lactate, dimerization and multimerization mixture:
[0042] The operation method is as in Example 1, 33g of cyclohexanol is added, the solvent is 50mL of benzene, the catalyst is 0.8g of p-toluenesulfonic acid, reflux for 3.5h, and the remainder is the same as in Example 1.
[0043] (2) Degradation of polymers:
[0044] At room temperature, dissolve the above-obtained mixture in 50 mL of absolute ethanol, add 1.0 g of solid sodium carbonate, stir for 2.5 h, GC detects dimers < 0.5%, filter with suction, and neutralize the filtrate with acetic acid to pH = 7.0, recover the solvent to obtain The oil was distilled under reduced pressure to obtain 45.3 g of the oil, and the purity of cyclohexanol lactate was 98.8% as detected by GC.
Embodiment 3
[0046] Prepare and purify lauryl lactate by the method of the present invention, comprise the following steps:
[0047] (1) Preparation of lauryl lactate, dimerization and multimerization mixture:
[0048] The operation method is as in Example 1, adding 60g of lauryl alcohol, the solvent is 50mL of petroleum ether (60-90°C), the catalyst is 5g of sodium bisulfate, reflux for 5.0h, and the remainder is the same as in Example 1.
[0049] (2) Degradation of polymers:
[0050] At room temperature, dissolve the above-obtained mixture in 75 mL of anhydrous methanol, add 0.6 g of solid potassium carbonate, stir for 2.5 h, GC detects that the dimer is <0.5%, filter with suction, and neutralize the filtrate with acetic acid to pH = 7.0, recover the solvent to obtain The oil was distilled under reduced pressure to obtain 75.2 g of the oil, and the purity of the lauryl lactate was 99.2% as detected by GC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com