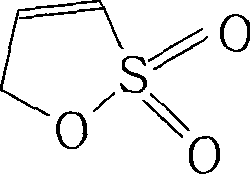
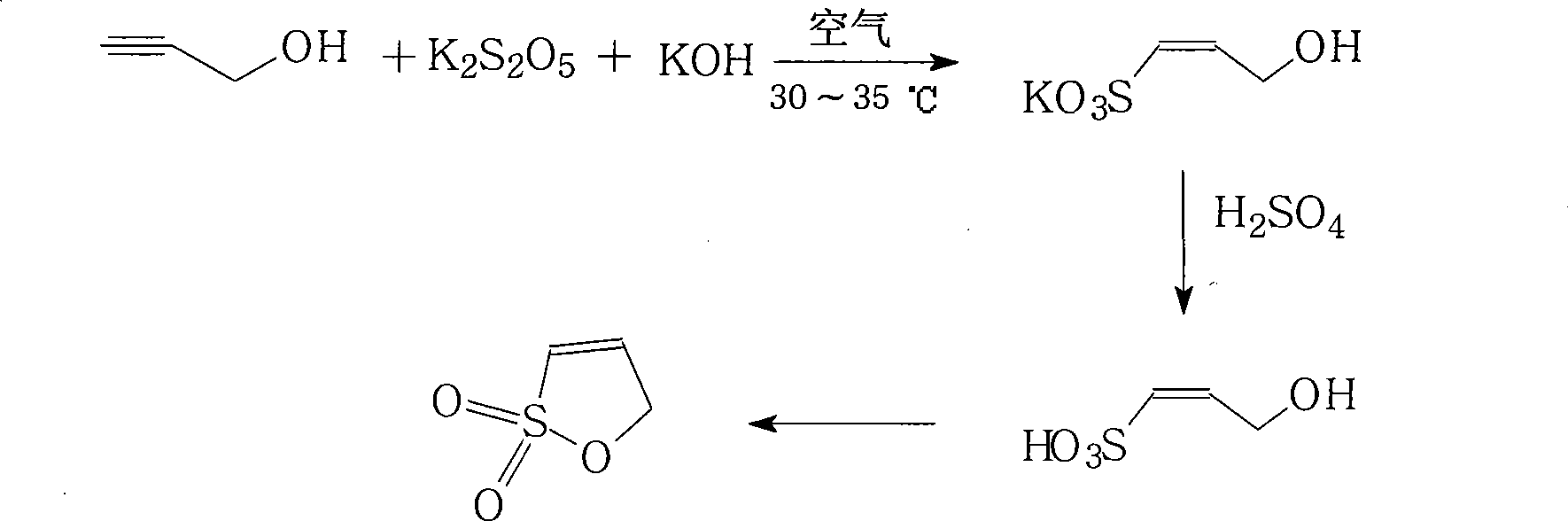
Method for preparing 1-propone-1,3-sultone
A technology of sultone and propylene, applied in the field of preparation of organic materials, can solve problems such as low yield and no industrial value, and achieve the effects of high product quality, low cost and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] A 500mL four-necked flask is equipped with a mechanical stirrer, an air inlet tube, and a dropping funnel. Put 50mL of propargyl alcohol and 100mL of water into the flask, and the temperature of the water bath is 30-35°C. Dissolve 20g of potassium bisulfite in 100mL of water to form a solution. Stirring was started, air was introduced at a rate of 10mL / min, and potassium bisulfite solution was added dropwise. After the dropwise addition was completed, the mixture was incubated and stirred for 3 hours.
[0062] Add 20mL of hydrochloric acid to the reaction system, concentrate with a rotary evaporator to the remaining 80mL, add 100mL of hydrochloric acid and 100mL of methanol, cool down to 10°C, and a large amount of crystals appear.
[0063] filter. The filtrate was concentrated until no liquid was evaporated, and the residue was heated to 120-130°C in an oil bath, reduced to 20mmHg, and kept ring-closed until anhydrous was evaporated.
[0064] The residue after cycli...
Embodiment 2
[0066] The reaction is the same as in Example 1. After the reaction is complete, add 30 mL of hydrochloric acid to the reaction system, concentrate to dryness with a rotary evaporator, place the residue in an oil bath and heat to 120-130° C., reduce the pressure to 20 mmHg, and keep warm until anhydrous and evaporate.
[0067] The residue after cyclization was extracted three times with 200mL×2 dichloromethane under reflux, the dichloromethane extracts were combined and concentrated, and a large number of white needle-like crystals were precipitated, filtered and recrystallized to obtain 6.4g of the product, with a yield of 32%, mp: 84~85℃, content: 99.9% (GC)
Embodiment 3
[0069] The reaction is the same as that in Example 1. After the reaction is complete, 30 mL of hydrochloric acid is added to the reaction system for acidification, and the obtained mixed solution is separated by column chromatography. The mobile phase is dichloromethane: methanol=1: 1, and the obtained 1-sulfonic acid-1-propenol After the solvent was evaporated from the solution, 12.5g of the product was obtained by direct high-vacuum ring-closure distillation, with a yield of 62.5%, mp: 84-85°C, content: 99.9% (GC)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com