Thioester in rhodamine B as well as preparation method and application thereof
A thioester and preparation process technology, applied in the field of mercury ion optical probes, can solve problems such as poor selectivity and influence, and achieve the effects of fast reaction speed, good solubility, and huge application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the preparation of rhodamine B lactone
[0018] The synthetic route of rhodamine B internal thioester is as follows:
[0019]
[0020] The specific synthesis process is as follows:
[0021] Step a): Dissolve 239mg of rhodamine B in about 5ml of 1,2-dichloroethane, slowly add about 0.3ml of phosphorus oxychloride, and react at reflux at 83°C for 4h. After cooling, the reaction system was vacuum-dried to obtain rhodamine B acid chloride.
[0022] Step b): Dissolve the obtained rhodamine B acid chloride in 6ml of tetrahydrofuran, then add the solution dropwise to 6ml of tetrahydrofuran solution containing 152mg of thiourea and 1.2ml of triethylamine, and stir overnight. The reaction system was evaporated to dryness under reduced pressure to obtain a purple-red oily liquid, and 5 ml of water was added thereto to produce a purple-red precipitate, which was washed with a large amount of water. The obtained crude product was separated by column chromatograp...
Embodiment 2
[0023] Embodiment 2, the spectroscopic properties of rhodamine B lactone and its reaction product with mercury ions
[0024] Weigh 4.6 mg of rhodamine B lactone to prepare 10 ml of 1,4-dioxane solution as mother solution (1 mM).
[0025] Add 50 μl of the above mother solution dropwise to a certain amount of 0.02M phosphate buffer solution, then add different equivalents of mercury ion solutions, and then use 0.02M phosphate buffer solution to make the volume to 10ml. After reacting at room temperature for 5 minutes, measure its UV-visible absorption spectrum and fluorescence emission spectrum. When the fluorescence emission spectrum is measured, it is de-excited at 530nm; the slit width for excitation and emission is 10nm; the voltage is 400V.
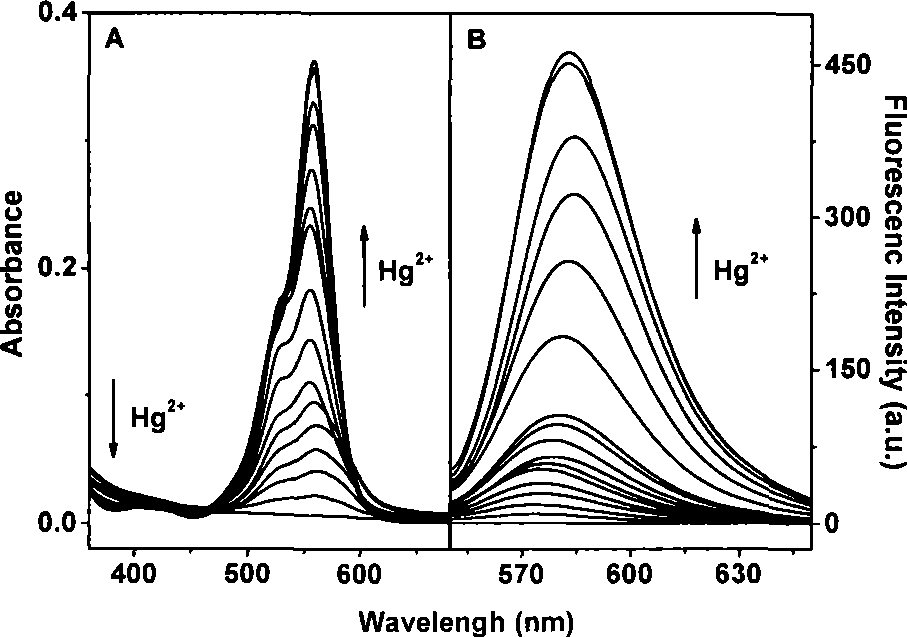
[0026] figure 1 It is the ultraviolet-visible absorption spectrum and fluorescence emission spectrum of 5 μM rhodamine B endothioate in the range of 0-100 μM final concentration of mercury ions.
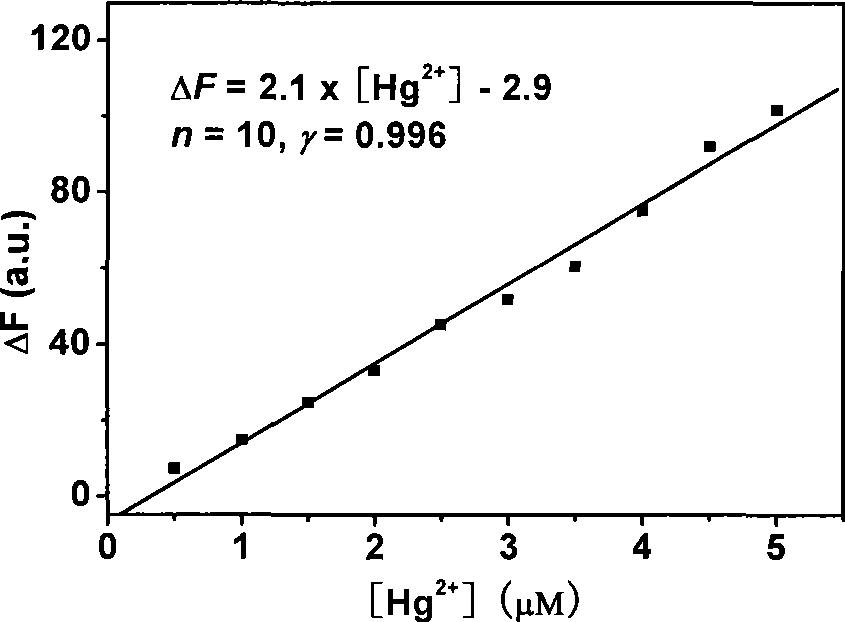
[0027] figure 2 It is the working...
Embodiment 3
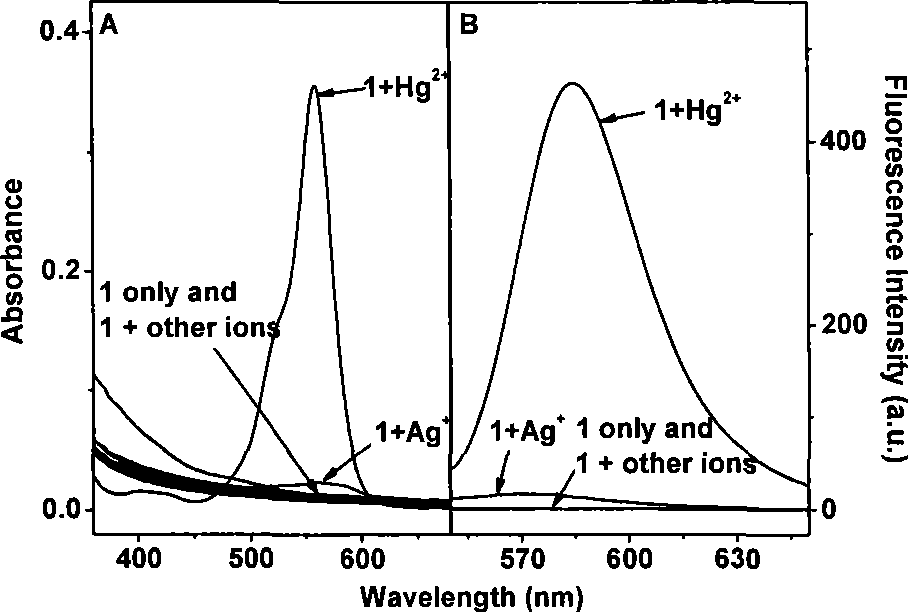
[0029] Example 3: Research on the interference of other metal ions on the determination of mercury by rhodamine B lactone.
[0030] Various metal ions (Ag + , Ca 2+ , Cd 2+ ,Co 2+ , Cu 2+ , Fe 3+ , K + , Mg 2+ , Mn 2+ , Pb 2+ , Zn 2+ or Hg 2+ ) to a final concentration of 50 μM. After reacting at room temperature for 5 minutes, measure its UV-visible absorption spectrum and fluorescence emission spectrum. When the fluorescence emission spectrum is measured, it is de-excited at 530nm; the slit width for excitation and emission is 10nm; the voltage is 400V.
[0031] Various metal ions (Ag + , Ca 2+ , Cd 2+ ,Co 2+ , Cu 2+ , Fe 3+ , K + , Mg 2+ , Mn 2+ , Pb 2+ , Zn 2+ ) solution, add 50 μl of rhodamine B lactone mother solution, and then add mercury ions to make the final concentration 50 μM.
[0032] image 3 Rhodamine B lactone (5μM) and various metal ions (Ag + , Ca 2+ , Cd 2+ ,Co 2+ , Cu 2+ , Fe 3+ , K + , Mg 2+ , Mn 2+ , Pb 2+ , Zn 2+ or Hg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com