Methods and means for nucleic acid sequencing
A nucleic acid sequencing and nucleic acid technology, applied in the field of determining the one or more reference sequences, possibly one or high-throughput sequencing, the sequencing disclosed in PCT/EP2005/002870, using reference sequences related to templates, can Solve the problems of huge sequencing burden and large CR to achieve effective analysis and identification, and cost-effective analysis and identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0143] The present invention advantageously uses rolling circle amplification to synthesize random arrays from a sample containing multiple template molecules in a single reaction. can be achieved up to 10 5 -10 7 per mm 2 Density. The random array synthesis method used in the embodiments of the present invention may include:
[0144] a. Provide a surface (eg glass) with an activated surface.
[0145] b. Attachment of primers, preferably via covalent bonds, or instead of covalent bonds, strong non-covalent bonds (eg biotin / streptavidin) can be used.
[0146] b. Addition of circular single-stranded template, preferably at a density suitable for the detection device.
[0147] c. Anneal the template to the primer.
[0148] d. Amplification using rolling circle amplification to generate long single-stranded tandem repeat templates that are attached to the surface at various locations.
[0149] (See eg Lizardi et al., for a description of "Mutation detection and single-molec...
Embodiment 1
[0237] PREPARATION OF DNA TEMPLATES FOR CANTALOUPE
[0238] enter
[0239] Double-stranded DNA template.
[0240] Template break:
[0241] Using the restriction enzyme CviJ I * (EURx, Poland), an enzyme that recognizes 5'-GC-3' and cuts blunt ends between them. Prepare the restriction reaction as follows:
[0242] 1 μg template 1.5 μg template 2 μg template 2x reaction buffer
25μl 2x reaction buffer
25μl 2x reaction buffer
25μl 0.3 units of CviJ I * 0.3 units of CviJ I * 0.3 units of CviJ I * Add water to 50μl Add water to 50μl Add water to 50μl total capacity
50μl total capacity
50μl total capacity
50μl
[0243] The reaction was incubated at 37°C for 1 hour.
[0244] The cleaved DNA was purified using a PCR cleanup kit (Qiagen) according to the manufacturer's protocol.
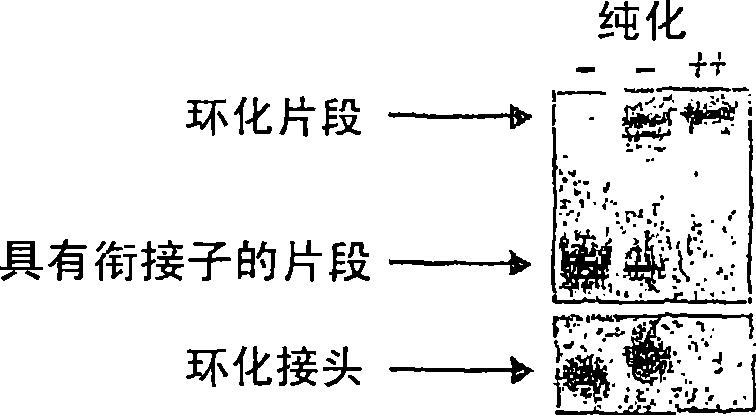
[0245] A fraction was analyzed on a 2% agarose gel to determine optimal reaction conditions for a particular batch of te...
Embodiment 2
[0281] Preparation of candidate region-enriched fragments for CANTALOUPE sequencing technology
[0282] Step 1: Selection of regions for enrichment and probe preparation
[0283] To enrich candidate regions of interest from nucleic acid samples, the following exemplary methods can be used prior to sequencing using Cantaloupe technology.
[0284] Based on genome-wide association studies of diseases or complex genetic traits such as Crohn's disease and psoriasis, the average candidate region size is approximately 500,000 bases (0.5 Mb). All candidate regions associated with the disease could be selected, but in this example, 3 different regions from different chromosomes were selected (H region: 453.5kb, R region: 285.5kb and E region: 193.6kb), which covered a total of 932.6 kb. Furthermore, in a separate example, only the E region (193.6 kb) was selected to verify the effect of size on the enrichment method of the present invention.
[0285] The probe sets used in this me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com