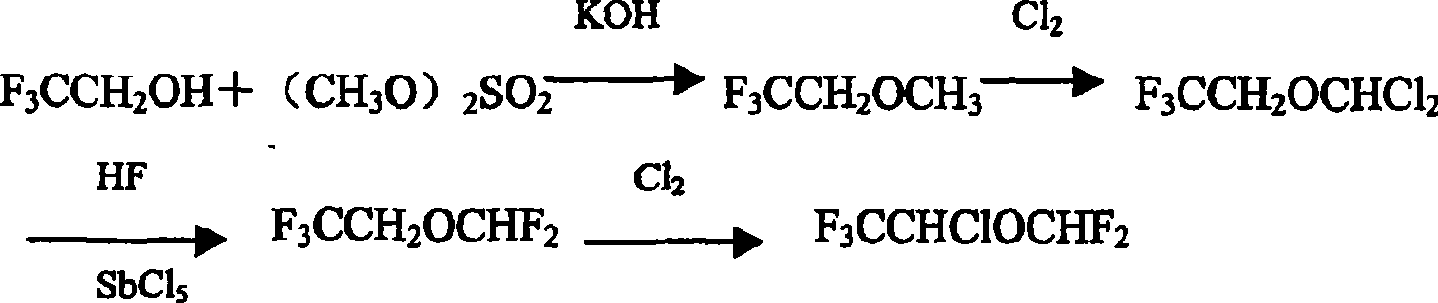
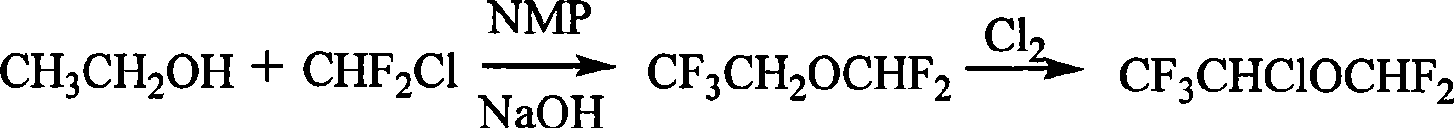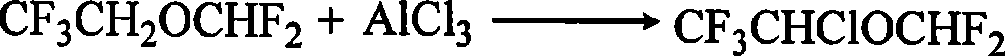Preparation of furane used as inhalation anesthetic
A technology of isoflurane and anesthetic, applied in the field of preparation of isoflurane, can solve the problems of high risk, high cost, low conversion rate, etc., and achieve the effect of low risk, low cost and small impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) 150gCCl 4 Add it into the reaction vessel, cool it in an ice bath to 0-5°C, add 135g (1mol) of anhydrous AlCl 3 , after stirring for 30 minutes, start to add 150gHFE (1mol) dropwise, control the drop rate, and keep the temperature in the system at 0-5°C; after the addition is completed, keep 20-40°C to continue the reaction for 24h;
[0032] (2) Cool the reaction product to 0-5°C, slowly add 333ml of 6mol / L hydrochloric acid dropwise, and leave to separate to obtain an oil phase;
[0033] (3) The oil phase is washed with 100ml of 2% sodium hydroxide solution, then washed with water (50 × 3ml) to neutrality, added anhydrous calcium chloride to dry, and after 24h, it is rectified with a 120 × 3cm packed column, 148 g of fractions at 42-48°C were collected and analyzed by MS-GC. The isoflurane content was 96.56%, and the yield was 77.5%.
Embodiment 2
[0035] (1) 150gCCl 4 Add it into a 500ml three-neck flask, cool it in an ice bath to 0-5°C, add 106.6g (0.8mol) of anhydrous AlCl 3 , after stirring for 35 minutes, start to add 150gHFE (1mol) dropwise, control the drop rate, keep the temperature in the system at 0-5°C, and keep the temperature at 20-40°C to continue the reaction for 12h after the addition is completed;
[0036] (2) Cool the reaction product to 0-5°C, slowly add 250ml of 6mol / L hydrochloric acid dropwise, and leave to separate to obtain an oil phase;
[0037] (3) The oily phase was washed with 100ml of 1% sodium hydroxide solution, then washed with water (50×3ml) until neutral, added anhydrous calcium chloride to dry, and after 12h, it was rectified with a 120×4cm packed column, and collected 137.4 g of fractions at 42-48°C were analyzed by MS-GC, the isoflurane content was 96.46%, and the yield was 73.6%.
Embodiment 3
[0039] (1) 300gCCl 4 Add it to a 1000ml three-necked flask with a reflux condenser, cool it to 0°C in an ice bath, and add 270 (2mol) of anhydrous AlCl 3 , after stirring for 25 minutes, start to add 150g HFE (1mol) dropwise, control the drop rate, keep the temperature in the system at 0-5°C, and keep the temperature at 20-40°C to continue the reaction for 24h after the addition is completed;
[0040] (2) Cool the reaction product to 0-5°C, slowly add 667ml of 12mol / L hydrochloric acid dropwise, and leave to separate to obtain an oil phase;
[0041] (3) The oil phase is washed with 200ml of 2% sodium hydroxide solution, then washed with water (100 × 3ml) to neutrality, added anhydrous calcium chloride to dry, and after 24h, it is rectified with a 120 × 3cm packed column, 142.4 g of fractions at 42-48°C were collected and analyzed by MS-GC. The isoflurane content was 96.42%, and the yield was 76.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com