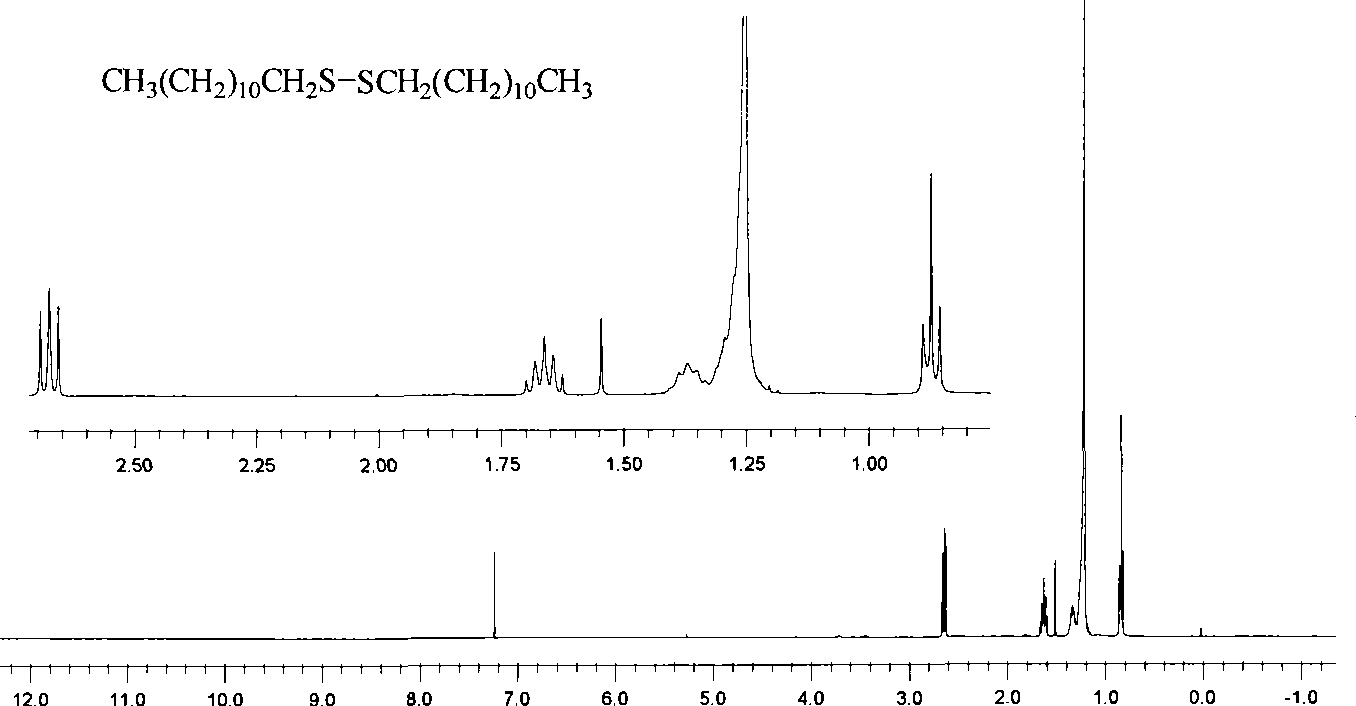
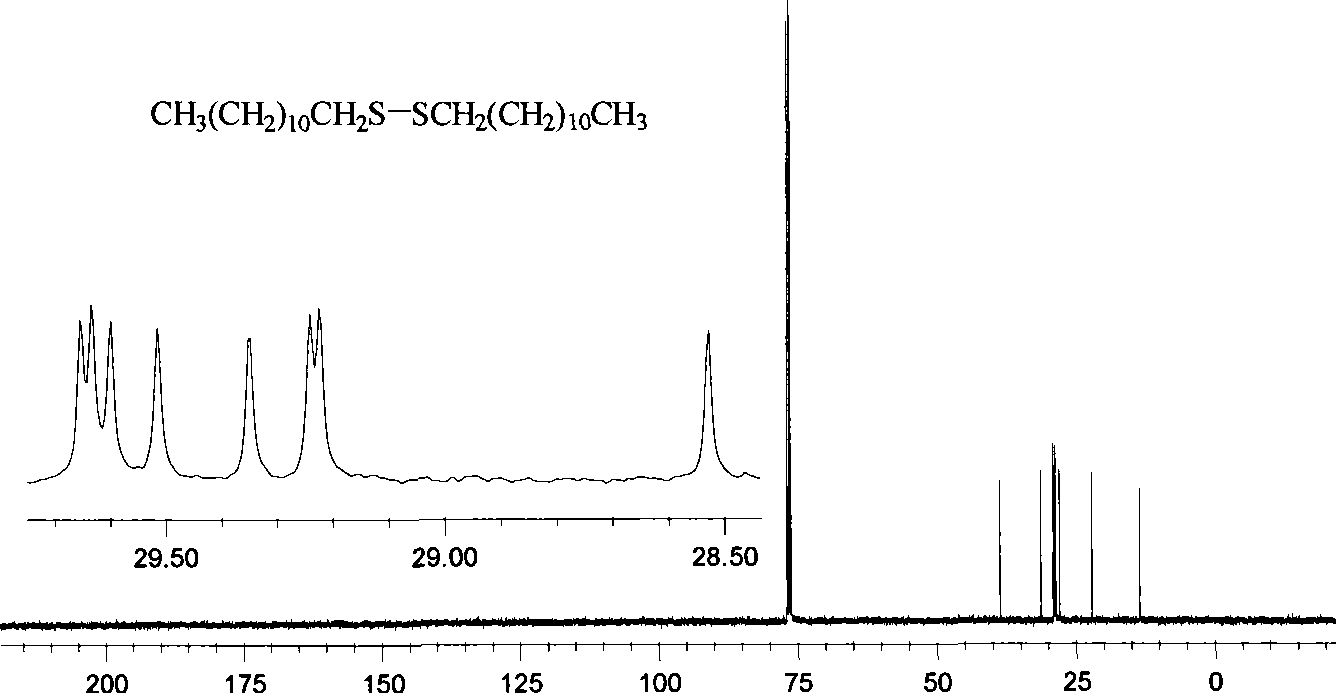
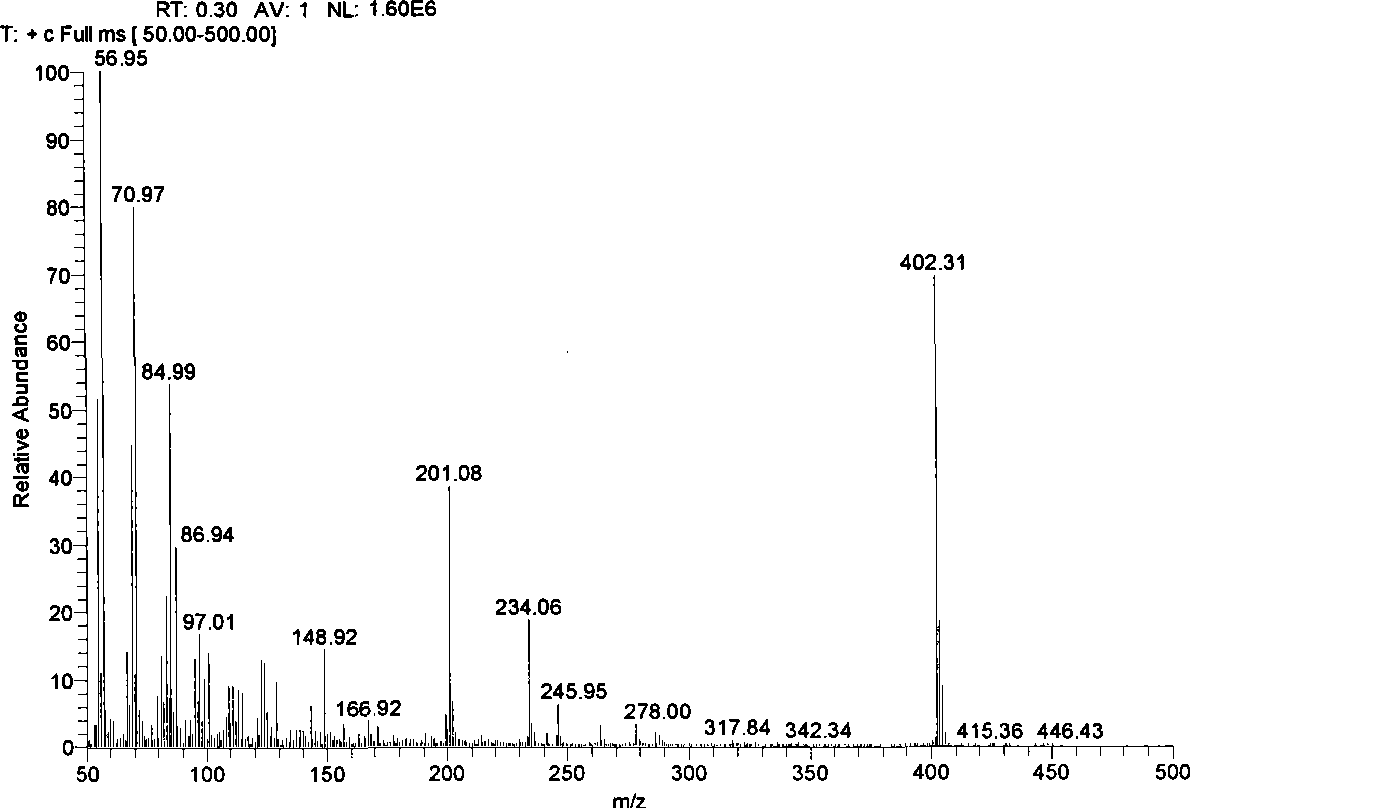
Preparation of symmetrical disulfide bond-bearing compound
A compound and disulfide bond technology, applied in the preparation of hydrogenated polysulfides/polysulfides, organic chemistry, etc., can solve the problems of expensive, complex, complicated reactions, etc., and achieve low cost, high yield, and simple and easy-to-obtain raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] n-dodecanethiol thiosulfur coupling reaction:
[0068] Add 0.200g (0.988mmol) of n-dodecanethiol into a 50mL Schlenk container, vacuum-dry for 45 minutes, fill with high-purity argon, add 10mL of anhydrous ether with a syringe, cool to -80~-75°C, Add 0.46mL n-hexane solution with a concentration of 2.57mol / L n-butyllithium dropwise with a syringe (the content of n-butyllithium is 1.1 equivalents), keep the reaction at -80~-75°C for 0.5 hours, add 0.399g (3 equivalents ) of anhydrous copper chloride, keep the reaction at -80~-75°C for 0.5 hours, slowly rise to -60~-55°C and keep the reaction for 1 hour, naturally rise to room temperature and keep the reaction for 8 hours; at 0~5°C Add water to quench the reaction, transfer the reaction system to a 250mL separatory funnel, separate the organic phase and the aqueous phase, extract the aqueous phase twice with 20mL ether, and combine the extract with the organic phase of the reaction product; separate the combined organic p...
Embodiment 2
[0070] Thiophenol sulfur sulfur coupling reaction:
[0071] Add 0.200g (1.82mmol) of thiophenol into a 50mL Schlenk container, vacuum-dry for 45 minutes, fill with high-purity argon, add 10mL of anhydrous ether with a syringe, cool to -80~-75°C, and use a syringe to Add dropwise 0.79mL n-hexane solution with a concentration of 2.73mol / L n-butyllithium (the content of n-butyllithium is 1.1 equivalents), keep the reaction at -80~-75°C for 0.5 hours, add 0.789g (3 equivalents) of Anhydrous copper chloride, keep the reaction at -80~-75°C for 0.5 hours, slowly rise to -60~-55°C and keep the reaction for 1 hour, naturally rise to room temperature and keep the reaction for 8 hours; add water at 0~5°C to quench To quench the reaction, transfer the reaction solution into a 250mL separatory funnel, separate the organic phase and the aqueous phase, extract the aqueous phase three times with 20mL ether, and combine the extract with the organic phase of the reaction product; separate the c...
Embodiment 3
[0073] 4-Methylthiophenol sulfur-sulfur coupling reaction:
[0074] Add 0.200g (1.61mmol) of 4-methylthiophenol into a 50mL Schlenk container, vacuum-dry for 45 minutes, fill with high-purity argon, add 10mL of anhydrous tetrahydrofuran with a syringe, and cool to -78~-73 ℃, use a syringe to drop 0.69mL n-hexane solution with a concentration of 2.57mol / L n-butyllithium (the content of n-butyllithium is 1.1 equivalent), keep the reaction at -78~-73℃ for 0.6 hours, add 0.649g ( 3 equivalents) of anhydrous copper chloride, keep the reaction at -78~-73°C for 0.6 hours, slowly rise to -58~-53°C and keep the reaction for 1.2 hours, naturally rise to room temperature and keep the reaction for 8.5 hours; Add water at 7°C to quench the reaction, transfer the reaction system to a 250mL separatory funnel, separate the organic phase and the aqueous phase, extract the aqueous phase twice with 20mL chloroform, and combine the extract with the organic phase of the reaction product; The phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com