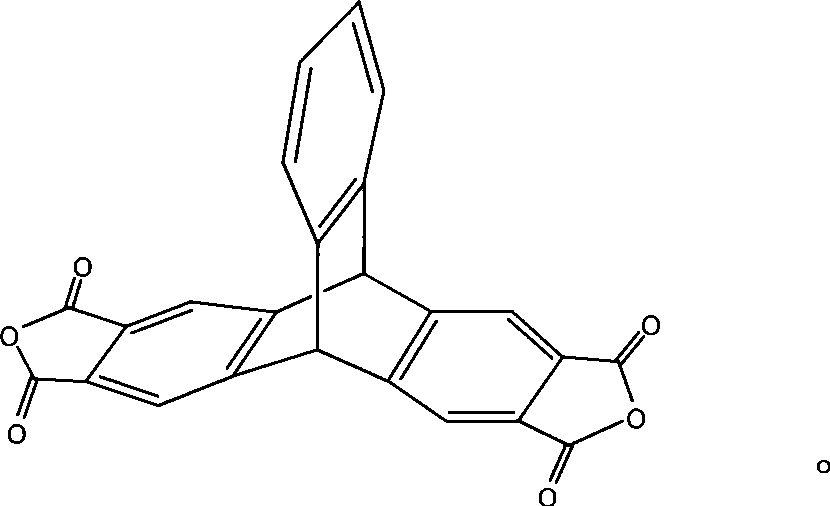
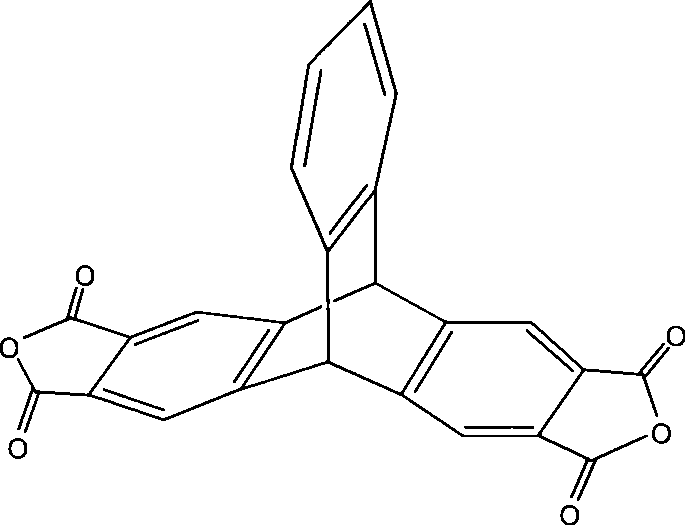
2,3,6,7-tetracarboxylic dianhydride triptycene and method for synthesizing the same
A technology of tetracarboxylic dianhydride triptycene and tetramethyl triptycene, applied in 2 fields, can solve problems such as difficulty in synthesizing polyimide, and achieve the effects of avoiding side reactions, easy production and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The following is the best embodiment of the invention
[0026] Under the conditions of ice-salt bath and vigorous stirring, 30g (grams) of aluminum chloride was divided into three additions and added in a 500ml three-necked flask equipped with 65ml (milliliters) of o-xylene and a mechanical stirrer, and then added 15g of phthalic anhydride in three batches, During the feeding process, ensure that the system temperature is lower than -5°C. After the addition, let the system stir at room temperature for 3 hours (hours), and then react in a water bath at 55° C. for 3 hours. Afterwards, slowly disperse the viscous mixed reactant in 500ml of 5% hydrochloric acid ice-water mixed solution. During the pouring process, vigorous stirring is required so that the excess aluminum chloride can be fully hydrolyzed. of white solid. Dissolve this solid in 300ml of 5% sodium hydroxide solution, let it stand for stratification, the upper layer is a small amount of o-xylene, and the lowe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com