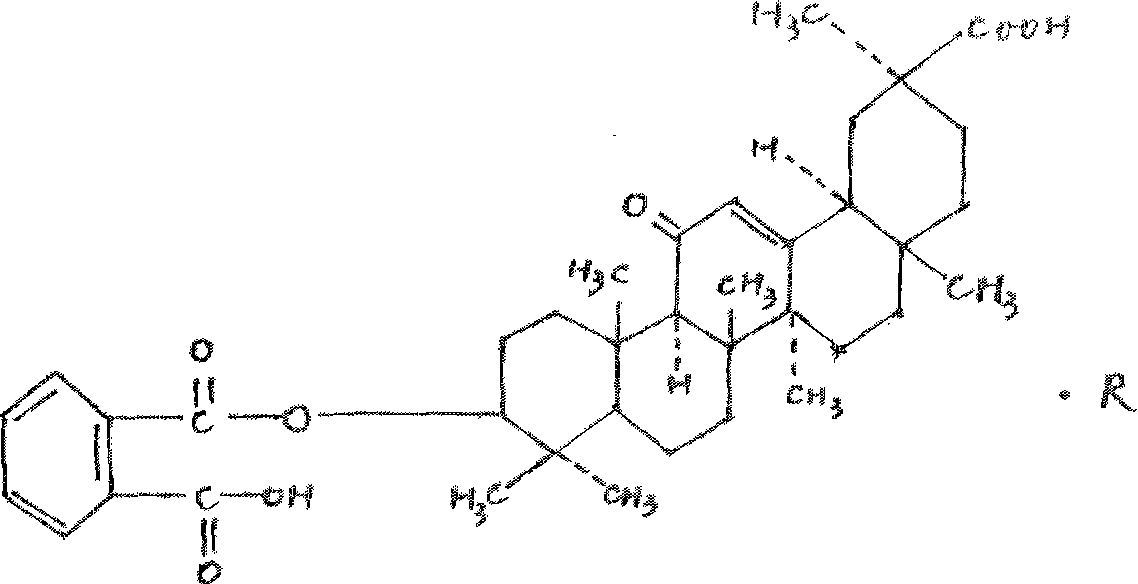
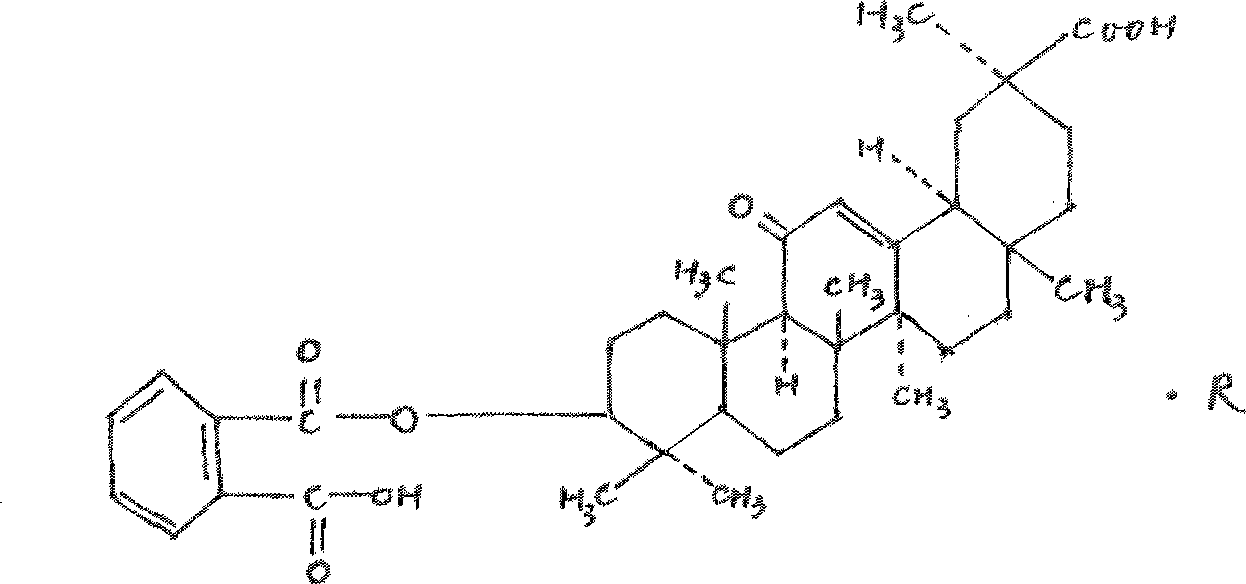
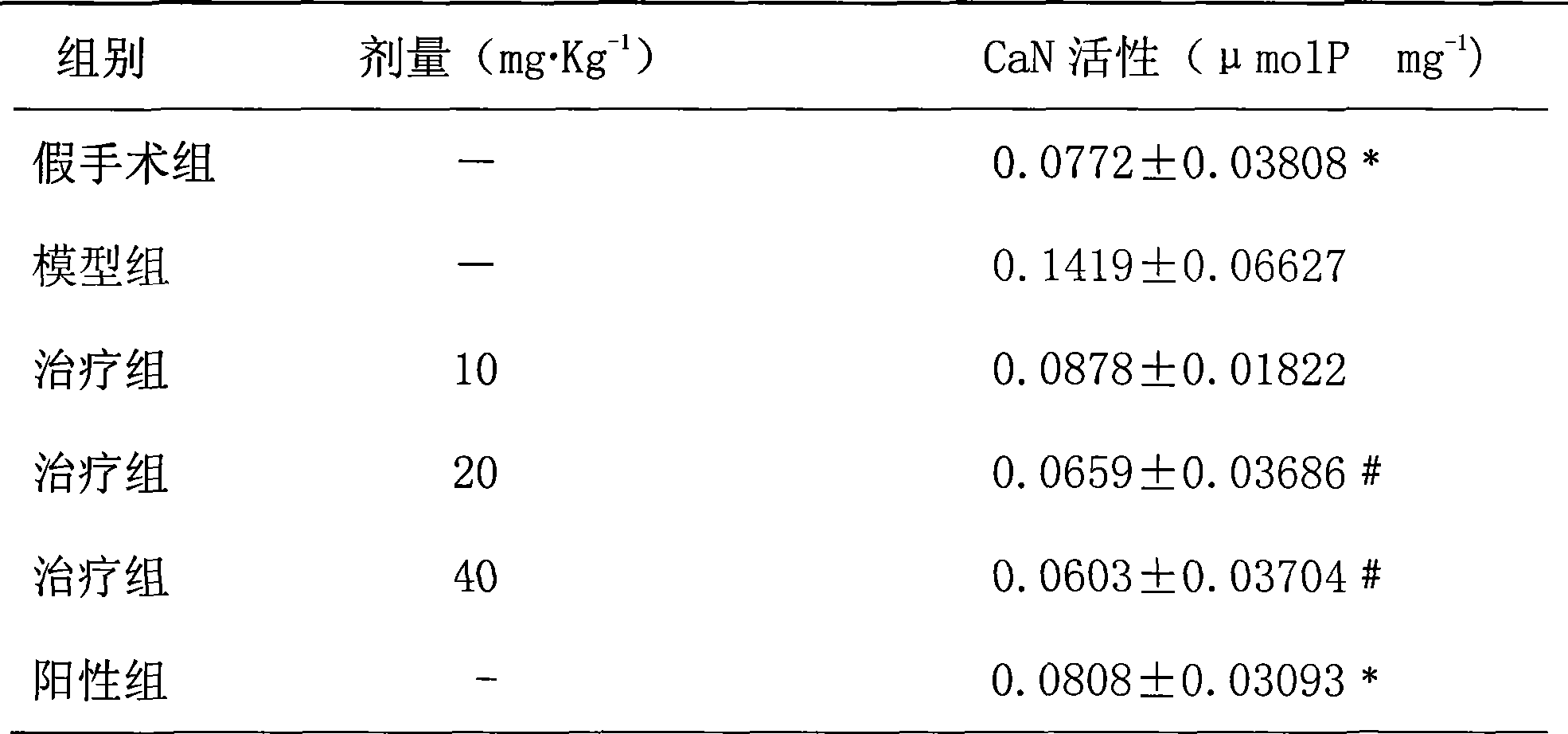
18 alpha-glycyrrhetinic o-phthalate, and preparation and use thereof
A technology of glycyrrhetic acid and phthalate, applied in pharmaceutical formulations, medical preparations containing active ingredients, digestive system, etc., can solve the problem of slow biotransformation, inability to produce inflammatory mediators, and inability to immediately exert curative effects, etc. problems, to achieve strong anti-inflammatory, reduce production costs, reduce tissue damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Weigh 823g (1.0mol) of 18β-glycyrrhizic acid produced by Xi'an Fujie Pharmaceutical Co., Ltd. and put it into a reaction tank, add 60L of 4mol / L sodium hydroxide, heat and reflux for 8 hours to obtain 576g of 18α-glycyrrhizic acid, The product yield is 69.99%.
[0036] [α] 20 =+18.0°(C=0.5 ethanol)
[0037] Elemental Analysis Results
[0038] Calculated value (%) Carbon: 61.30 Hydrogen: 7.59
[0039] Measured value (%) Carbon: 61.42 Hydrogen: 7.53
[0040] The 18α-glycyrrhizic acid that takes by weighing 823g (1.0mol) is put into reaction tank, first adds 80L water and stirs to dissolve, then adds hydrogen type sulfonic acid cationic resin catalytic hydrolysis, wherein the add-on of hydrogen type sulfonic acid cationic resin is 18α- 50 times the volume of glycyrrhizic acid. Then heat the reaction tank to 98°C, stir and reflux for 9 hours, filter to remove the resin, and concentrate the filtrate under reduced pressure; add ethanol until the alcohol content is 40%...
Embodiment 2
[0049] Weigh 823g (1.0mol) of 18β-glycyrrhizic acid produced by Xi'an Fujie Pharmaceutical Co., Ltd. and put it into a reaction tank, add 80L of 4mol / L sodium hydroxide, and heat and reflux for 10 hours to obtain 576g of 18α-glycyrrhizic acid. The rate is 69.99%.
[0050] Weigh 823g (1.0mol) of the prepared 18α-glycyrrhizic acid into a reaction tank, first add 80L of water and stir to dissolve, then add 8L of sulfuric acid with a concentration of 50%, and bathe in boiling water for 5 hours. Filter, wash the precipitate with distilled water until the pH is close to 7, and dry at 105°C to obtain 350 g of 18α-glycyrrhetinic acid with a yield of 74.4%.
[0051] Weigh 0.5g of the prepared 18α-glycyrrhetinic acid and put it into the reaction tank, first add phthalic anhydride, wherein the molar ratio of 18α-glycyrrhetinic acid to the added phthalic anhydride is 1:30; then add 7ml of pyridine to dissolve, boil water bath Reflux for 50 hours. After the reaction is complete, dilute hy...
Embodiment 3
[0055] Weigh 823g (1.0mol) of 18β-glycyrrhizic acid produced by Xi'an Fujie Pharmaceutical Co., Ltd. and put it into a reaction tank, add 80L of 4mol / L sodium hydroxide, and heat and reflux for 12 hours to obtain 576g of 18α-glycyrrhizic acid. The rate is 69.99%.
[0056] Weigh 823g (1.0mol) of the prepared 18α-glycyrrhizic acid into a reaction tank, first add 80L of water and stir to dissolve, then add 10L of 50% hydrochloric acid, and bathe in boiling water for 8 hours. Filter, wash the precipitate with distilled water until the pH is close to 7, and dry at 105° C. to obtain 330 g of 18α-glycyrrhetinic acid, with a product yield of 70.1%.
[0057] Weigh 0.5 g of the prepared 18α-glycyrrhetinic acid and put it into the reaction tank, first add phthalic anhydride, wherein the molar ratio of 18α-glycyrrhetinic acid to the added phthalic anhydride is 1:20; then add 7ml of N,N- Dimethylformamide was dissolved, and the boiling water bath was refluxed for 30 hours. After the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com