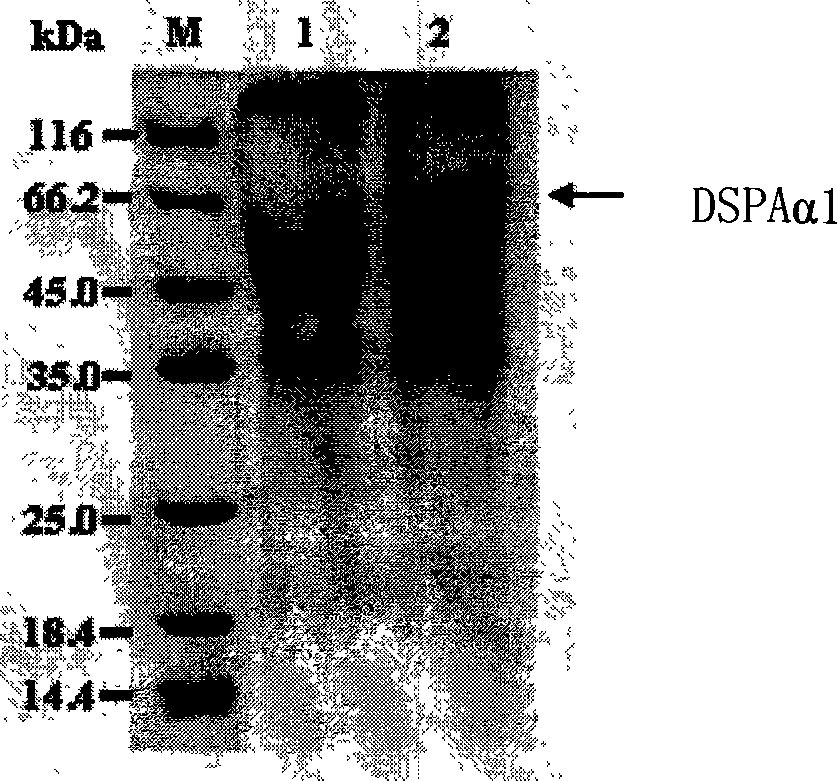
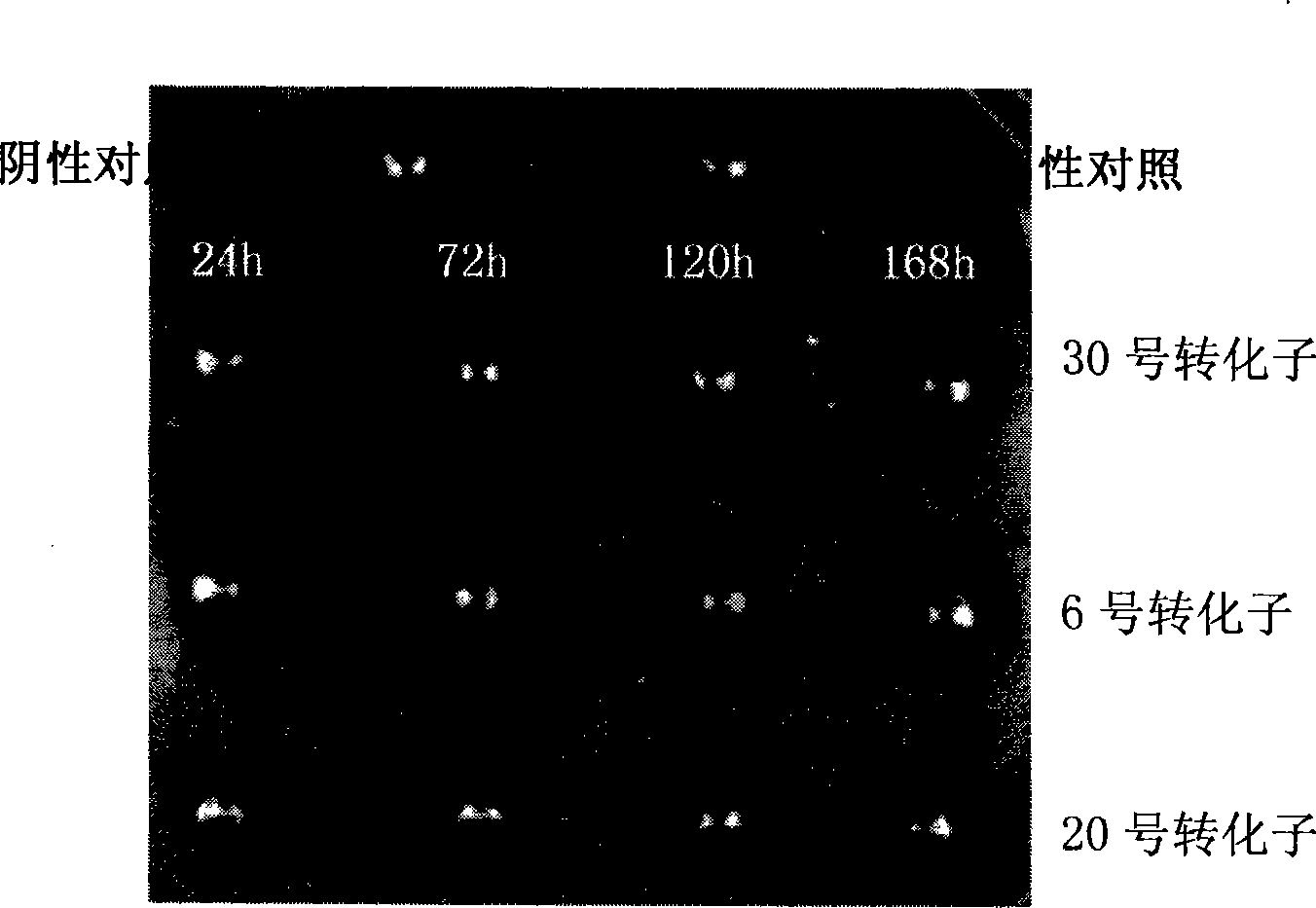
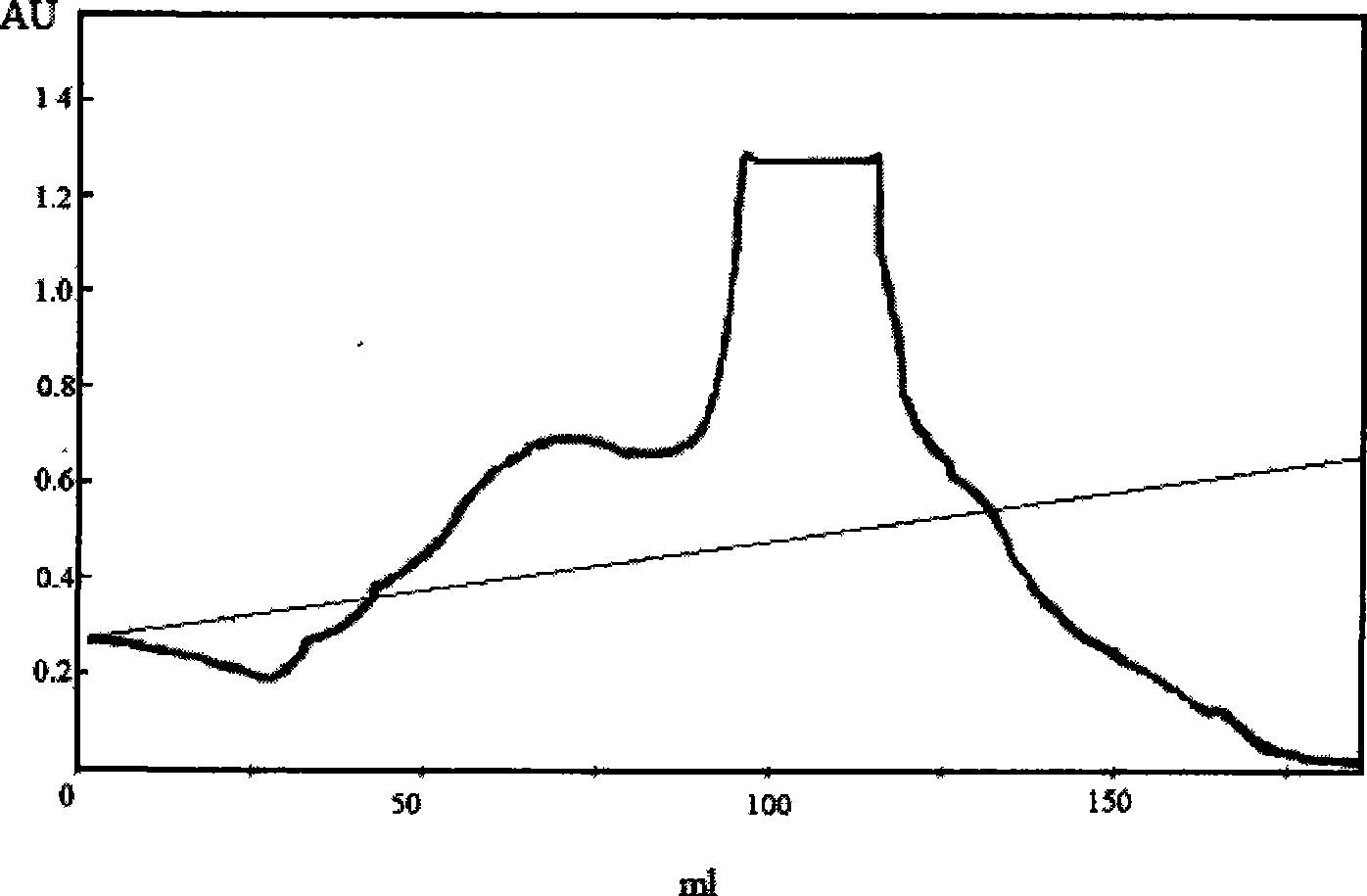
Method for producing DSPA alpha 1 by yeast expression system
An expression system, the technology of Pichia pastoris, applied in the field of biomedicine, can solve the problems of unsuitability for industrial production and no successful expression of DSPAα1, and achieve the effect of low cost, suitable for industrial production, and easy technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Gene synthesis of DSPAα1 gene and Pichia pastoris preferred codon sequence
[0050] Synthesize the nucleotide sequence of the DSPAα1 gene or optimize the nucleotide sequence of the DSPAα1 gene according to the preferred codon sequence of Pichia pastoris.
[0051] On the basis of not changing the amino acid sequence of DSPAα1, the whole gene sequence of DSPAα1 was synthesized according to the preferred codons of Pichia pastoris, and the XhoI restriction site and the cleavage signal amino acid codon ( AAAAGA), while introducing EcoRI or NotI restriction sites at the 3' end, the nucleotide sequence of the synthetic Pichia pastoris preferred codon-optimized DSPAα1 gene is shown in SEQ ID NO.1 (synthetic work provided by Shanghai Sangon Bioengineering Technology Services Co., Ltd.).
[0052] 2. Construction of recombinant plasmids
[0053] The steps are as follows: use restriction endonuclease XhoI and EcoRI to double digest the DSPAα1 synthetic gene and pPIC9 expressio...
Embodiment 2
[0077] 1. Gene synthesis of DSPAα1 gene and Pichia pastoris preferred codon sequence
[0078] Step is with embodiment 1.
[0079] 2. Construction of recombinant plasmids
[0080] The constructed pPIC9K-DSPAα1 was digested with XhoI and NotI, and the fragment containing DSPAα1 was recovered and ligated with the expression plasmid pPICZαA digested with the same enzyme using T4 DNA ligase to construct the recombinant plasmid pPICZαA-DSPAα1.
[0081] 3. Pichia pastoris strain transformation and plate screening
[0082] SalI linearized the plasmid pPICZαA-DSPAα1 and the blank plasmid pPICZαA, and took 5 μg to 20 μg of the plasmid to transform Pichia pastoris GS115 competent cells by electroporation.
[0083] The parameters of the GenepulsterII electroporation instrument (Bio-Rad laboratories Inc., Hercules, CA) used were: voltage 1.5kV, capacitance 25μF, resistance 200Ω, and discharge time 4.0ms. Immediately after electric shock, add 1.0 mL of ice-cold 1.0 mol / L sorbitol, and in...
Embodiment 3
[0094] 1. Gene synthesis of DSPAα1 gene and Pichia pastoris preferred codon sequence
[0095] Step is with embodiment 1.
[0096] 2. Construction of recombinant plasmids
[0097] The constructed pPIC9K-DSPAα1 was digested with XhoI and NotI, and the fragment containing DSPAα1 was recovered and ligated with the expression plasmid pGAPαA digested with the same restriction enzyme using T4 DNA ligase to construct the recombinant plasmid pGAPαA-DSPAα1.
[0098] 3. Pichia pastoris strain transformation and plate screening
[0099] The SalI linearized plasmid pGAPαA-DSPAα1 and the blank plasmid pGAPαA, the transformation of Pichia pastoris strain GS115 and the plate screening are the same as in Example 2 for plate screening.
[0100] 4. Fermentation screening of Pichia pastoris recombinant strains
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com