Carbazole organic semiconductor materials, methods for preparing and using same
A technology of organic semiconductors and application methods, applied in the fields of semiconductor/solid-state device manufacturing, semiconductor devices, organic chemistry, etc., can solve problems such as lack of synthesis methods, and achieve the effect of simple synthesis routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
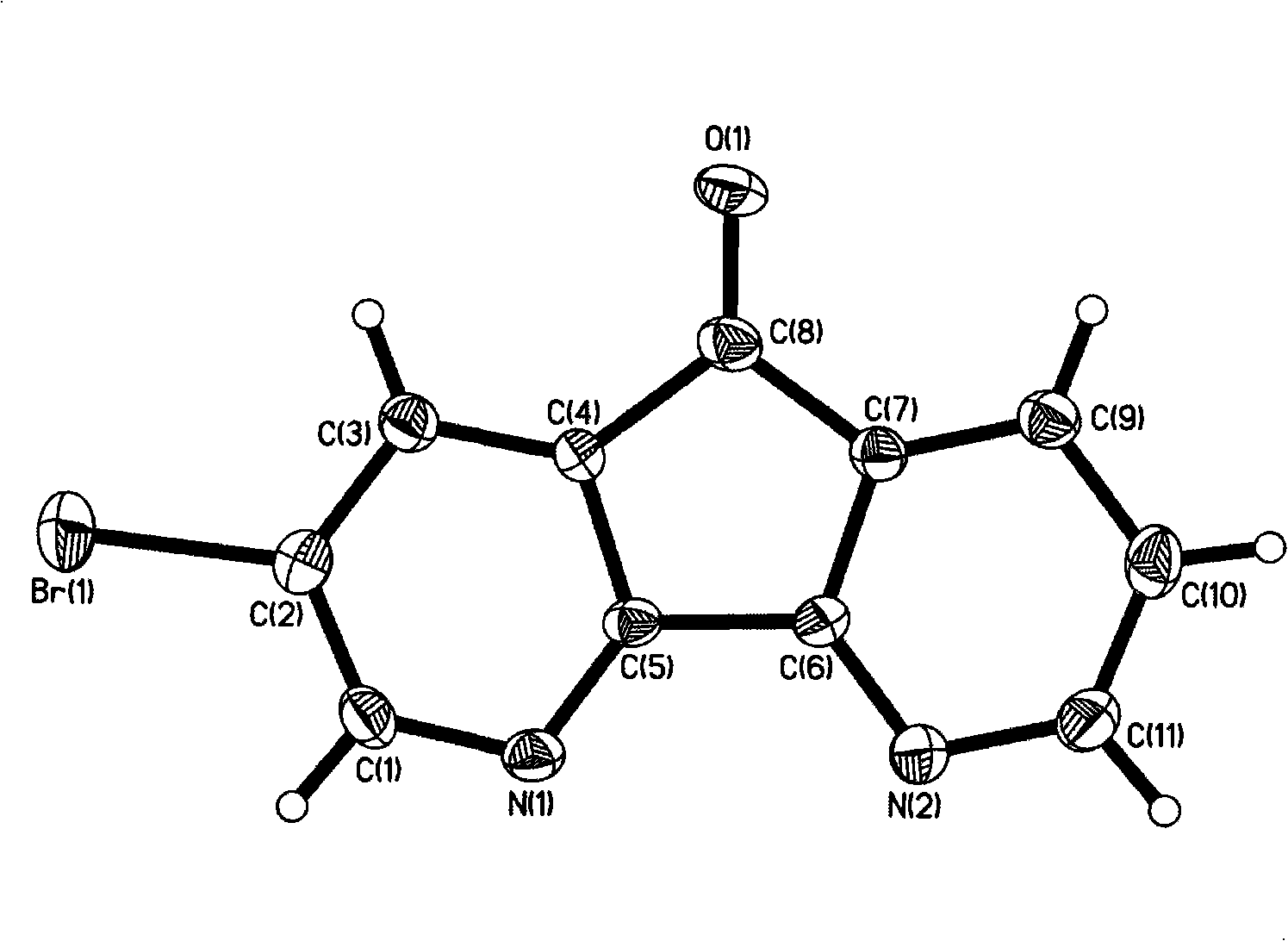
[0047] A method for preparing an azafluorene-based organic semiconductor material. The compound material I is prepared from 2-bromoazafluorenone or 2-iodoazafluorenone, and has the following structure:
[0048]
[0049] Its 2-bromoazafluorenone synthesis method is as follows: its 2-bromoazafluorenone synthesis method is as follows: take phenanthroline and KBr and place them in a 100ml single-necked flask, carefully add the mixed concentrated nitric acid and concentrated sulfuric acid to the reaction flask , the temperature is between 80-170 degrees, and the reflux time is 5-24 hours. After the reaction, put the reaction solution into ice water, neutralize, filter, extract the filtrate, combine the organic layers, and anhydrous MgSO 4After drying, the solvent was removed by rotary evaporation to obtain a crude product, which was subjected to column chromatography using a mixed solvent of petroleum ether and ethyl acetate as an eluent to obtain a light yellow solid product wit...
Embodiment 1
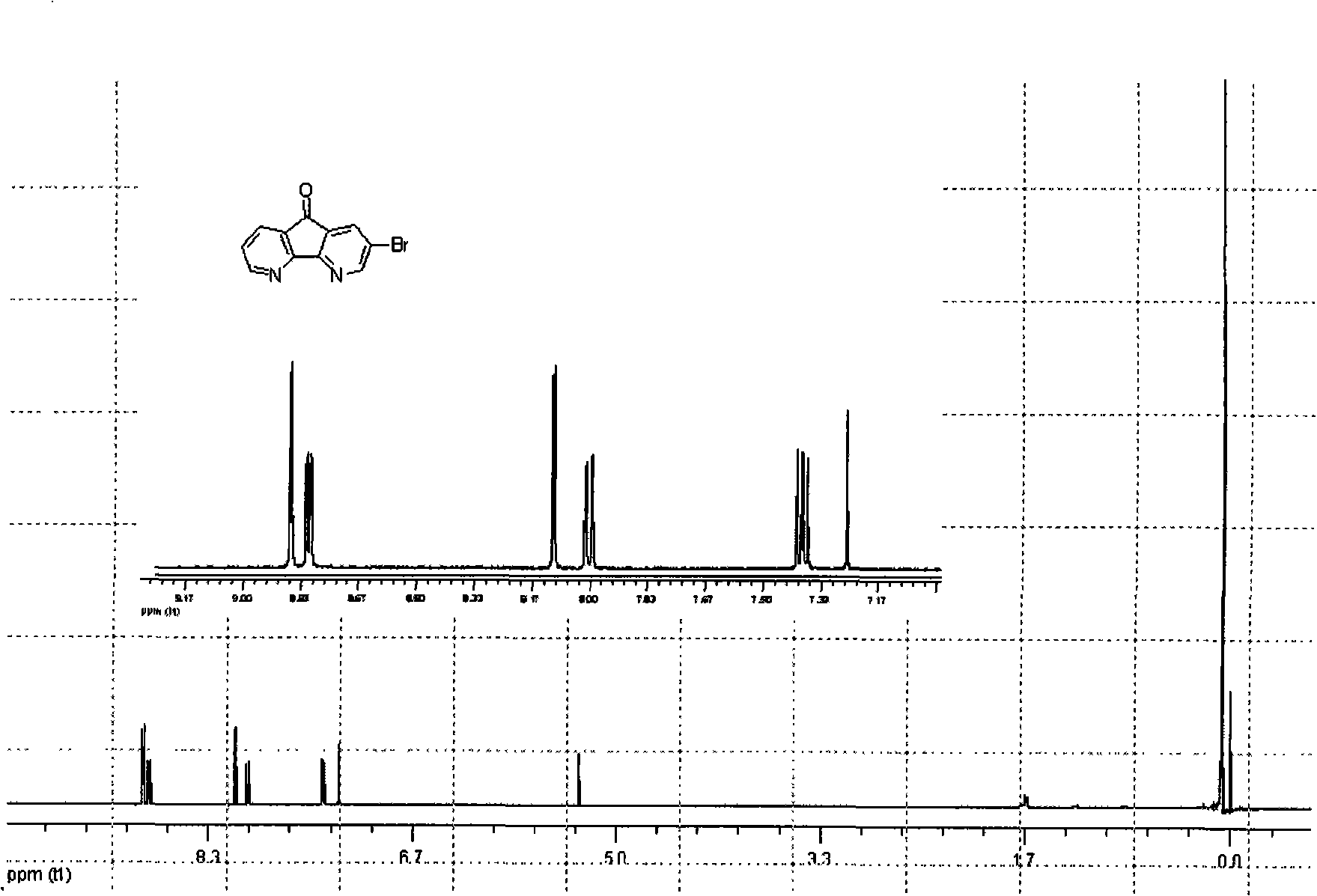
[0058] The preparation of embodiment 1,2-bromo azafluorenone:
[0059]
[0060] Get phenanthroline (2.93g, 14.8mmol) and KBr (2.11g, 17.8mmol) and place in a 100ml single-necked flask, carefully add the mixed concentrated nitric acid and concentrated sulfuric acid (18mL and 36mL) into the reaction flask, and heat to 100 ℃, keep reflux for 12 hours, after the reaction, pour the reaction solution into ice water, use NaOH solid to neutralize the solution to pH 7, filter, and use CHCl 3 The soluble solid was washed into the filtrate and washed with CHCl 3 Extract the filtrate, combine the organic layers, anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized from water to obtain a light yellow solid product with a yield of 50%.
Embodiment 2、2
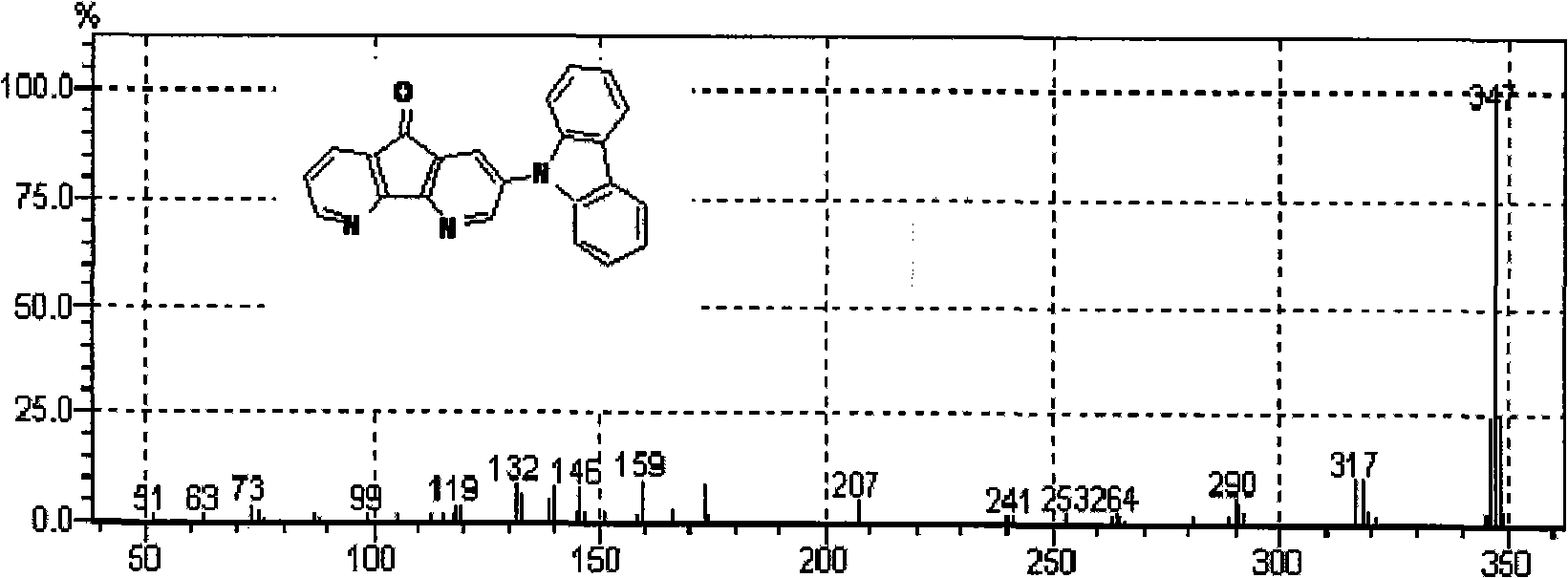
[0061] The preparation of embodiment 2,2-carbazole azafluorenone:
[0062]
[0063] Add carbazole (0.7401g, 4.4262mmol), 2-bromoazafluorenone (0.5012g, 1.9203mmol), potassium carbonate (0.3197g, 2.3131mmol), cuprous iodide (0.0803g) in a 50ml round bottom flask , 0.4216mol), small pieces of 18-crown-6 and dichlorobenzene (26ml), the reaction system was refluxed for 24 hours under the protection of nitrogen. Then, the dichlorobenzene in the system was distilled off under reduced pressure, and the remaining product was extracted with dichloromethane, and the organic phases were combined, anhydrous MgSO 4 After drying, the solvent was removed by rotary evaporation to obtain a crude product, which was subjected to column chromatography using a mixed solvent of petroleum ether and ethyl acetate as an eluent to obtain a yellow product with a yield of 71%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com