Preparation of 3-(3,4-dihydroxyphenyl)-acrylic acid 2-(3,4-dihydroxyphenyl)-ethyl ester and derivative phenyl acrylic acid phenyl alkyl ester compound
A technology of phenylalkenyl phenylacrylate and compounds, which is applied in the field of caffeic acid 3, phenylalkenyl phenylacrylate compounds, and can solve the problems of difficult separation of target compounds, many reaction by-products, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
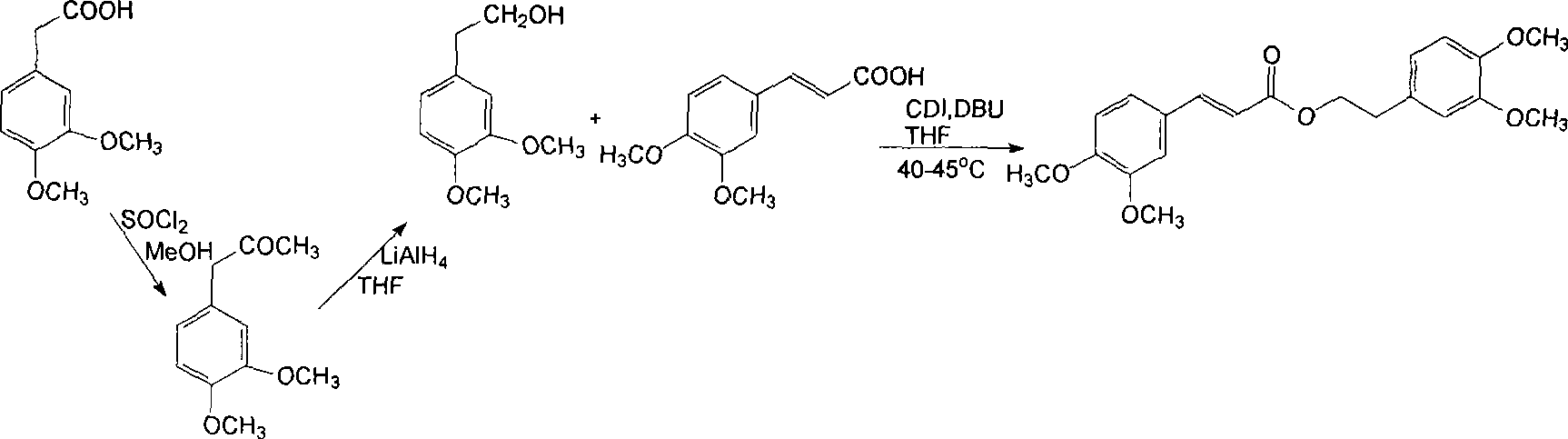
Image
Examples
Embodiment 1
[0162] Preparation of Meldrum's acid
[0163] Slowly add concentrated sulfuric acid to the mixture of malonic acid and acetic anhydride and keep stirring until most of the malonic acid is dissolved, then add acetone and keep the temperature at 20-25°C. The reactant was placed in a refrigerator at 4°C overnight until crystals were precipitated, and the filtered crystals were washed three times with ice water to obtain Meldrum's acid. The ratio of malonic acid, acetic anhydride, acetone and concentrated sulfuric acid used in the reaction is 1.0(mol):1.2~1.5(mol):1.1~1.3(mol):5~8(ml).
[0164] 1 H-NMR (MeOH-d 4 , 600MHz) δ 1.77 (6H, s, -CH 3 ), 3.85 (2H, s, -CH 2 -);
[0165] 13 C-NMR (MeOH-d 4 , 150MHz) δ 27.7 (q, -CH 3 ), 37.0(t, -CH 2 -), 107.6 (s, O-C-O), 165.7 (s, -C=O).
Embodiment 2
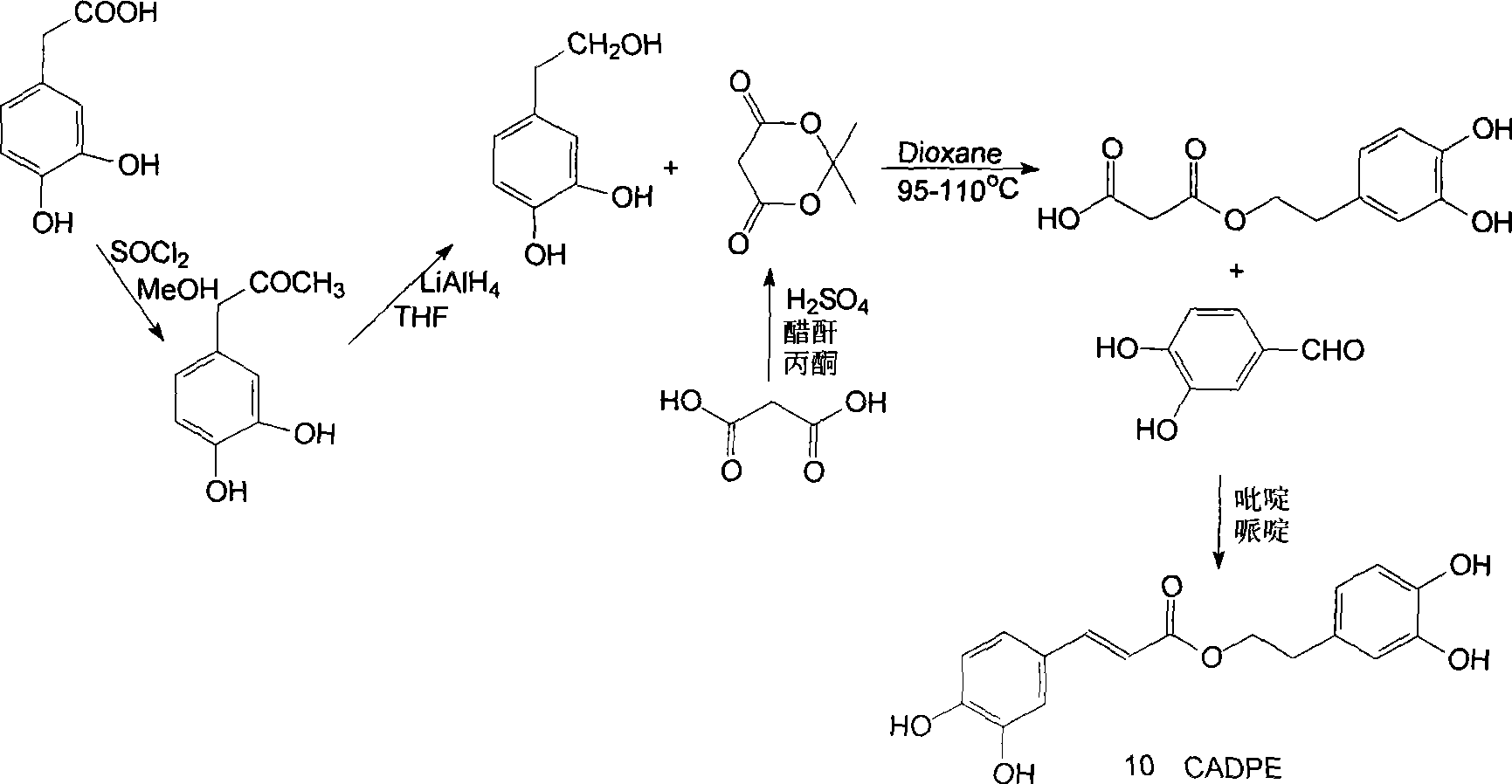
[0167] Preparation of CADPE: the synthetic route is shown in the appendix figure 1
[0168] 1) Preparation of 3,4-dihydroxyphenylacetic acid methyl ester: 3,4-dihydroxyphenylacetic acid (336.3 grams, 2.0mol) was dissolved in 4000mL of anhydrous methanol, and gradually added thionyl chloride ( 174.0 mL, 2.4 mol) and stirred for 15 minutes, then left at room temperature for 7-8 hours without constant stirring. The reactant was concentrated under reduced pressure to obtain a residue, and the residue was eluted with a mixed solvent of cyclohexane and ethyl acetate (4:1) through silica gel column chromatography. (TLC) detects each component under the ultraviolet light, will contain 3, after the eluent of 4-dihydroxyphenylacetic acid methyl ester is combined, reclaim solvent and obtain 3, 4-dihydroxyphenylacetic acid methyl ester (332.2 grams, yield 91.2%).
[0169] 1 H-NMR (MeOH-d 4 , 600MHz) δ 3.45 (2H, s, H-7), 3.64 (3H, s, OCH 3 ), 6.55 (1H, dd, J=2.0 / 8.0Hz, H-6), 6.69 (1...
Embodiment 3
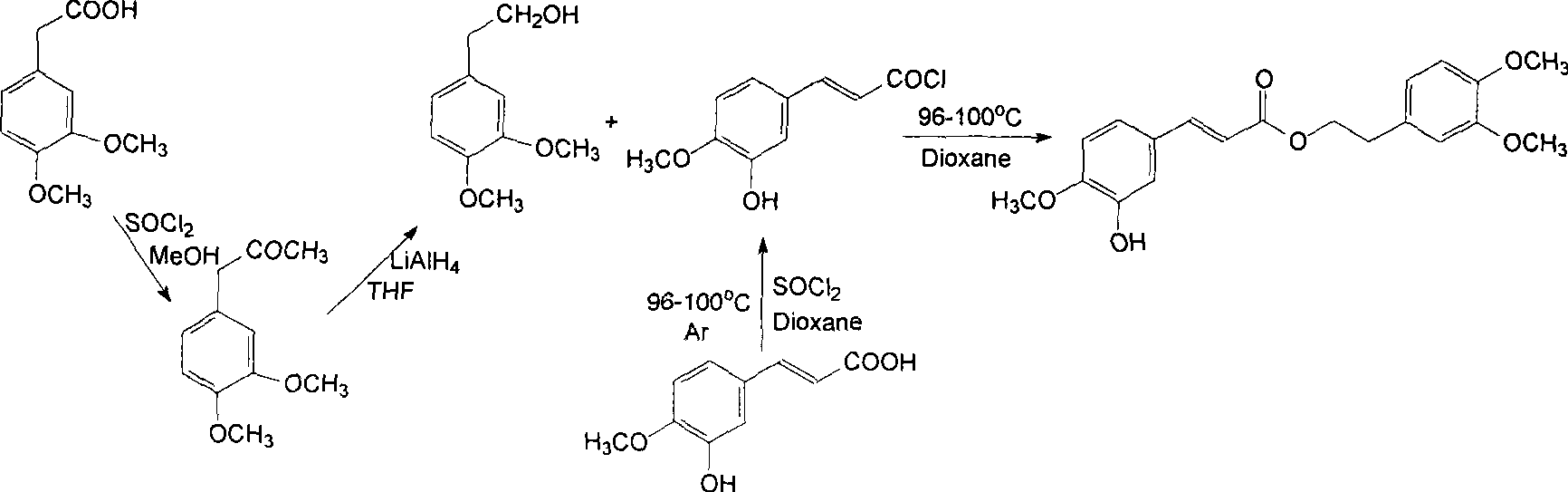
[0184] Preparation of 2-(3,4-diacetoxyphenyl)ethyl 3-(3,4-diacetoxyphenyl)-allylic acid ester
[0185] Dissolve 3,4-dihydroxyphenethyl caffeic acid (prepared in Example 2, 1.0 g) in a mixed solvent (50 mL) of pyridine and acetic anhydride (2:1), leave it at room temperature overnight, add a small amount of distilled water, and wait for the reaction The residue was naturally cooled and concentrated under reduced pressure to obtain a residue. The residue was separated and purified by silica gel column chromatography (eluted with a mixed solvent of cyclohexane and ethyl acetate) to obtain the target compound (1.39 g).
[0186] The molecular formula of this compound is C 25 h 24 o 10 , Colorless powder, soluble in methanol, ethyl acetate and chloroform.
[0187] 1 H-NMR data (MeOH-d 4 , 600MHz) δ 2.19, 2.20, 2.21, 2.22 (each 3H, s, OAc-3, 4, 3′, 4′), 2.96 (2H, t, J=6.8Hz, H-7′), 4.36 (2H , t, J=6.8Hz, H-8'), 6.42(1H, d, J=15.9Hz, H-8), 7.03(1H, d, J=8.0Hz, H-5), 7.12(1H, d,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com