Catalyst for preparing 1,4-butanediol or tetrahydrofuran from selective hydrogenation of dimethyl maleate and preparation method thereof
A technology of dimethyl maleate and selective hydrogenation, which is applied in the chemical industry, can solve the problem of a small adjustment range of the composition of the hydrogenation product, and achieves good industrial application prospects and the effect of enhancing elasticity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
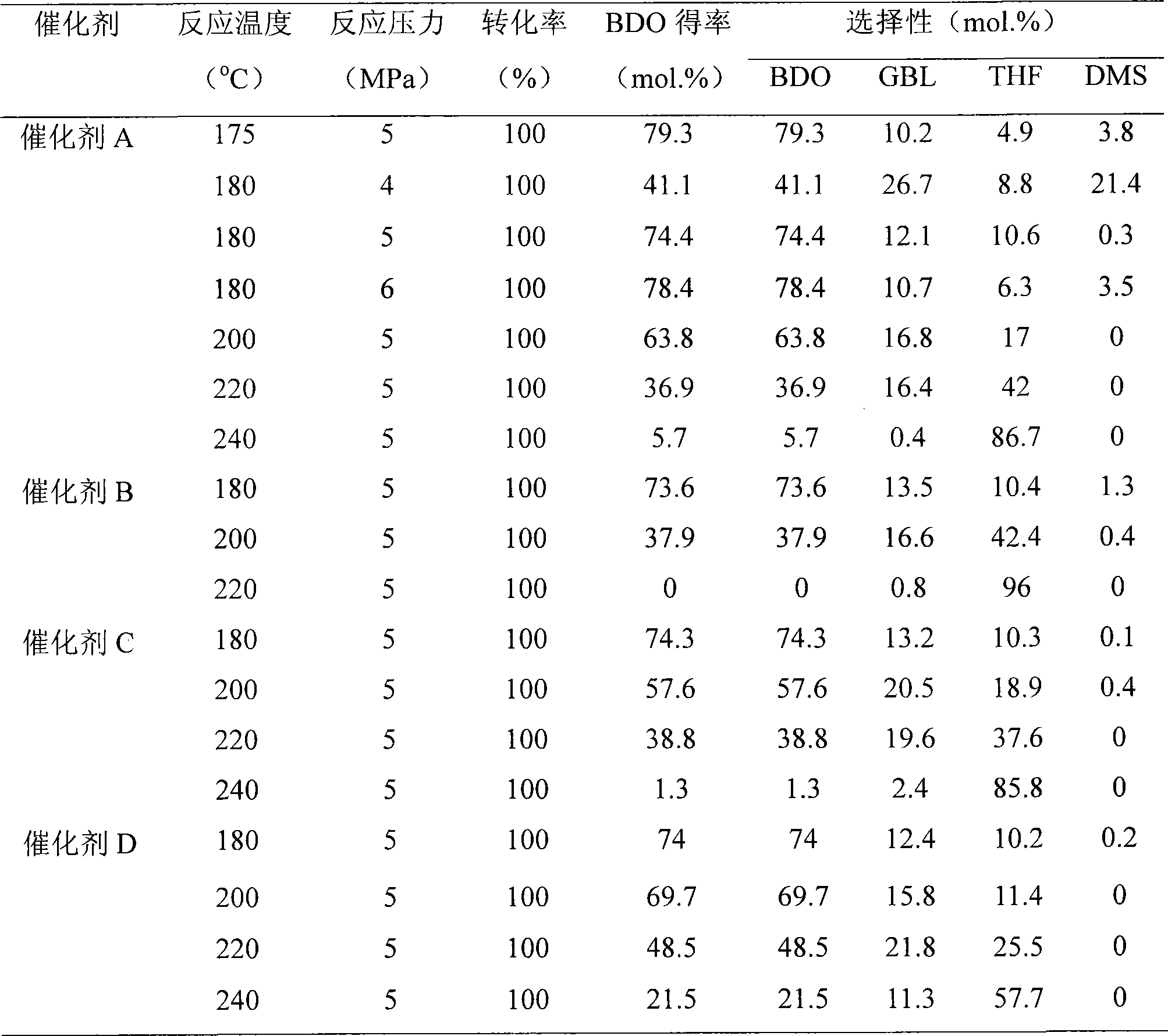
[0020] 10.0g Cu(NO 3 ) 2 ·3H 2 O, 9.8g Zn(NO 3 ) 2 ·6H 2 O dissolved in 75ml H 2 O is made into a metal ion solution, 8.7gNa 2 CO 3 Dissolved in 82ml H 2 O to obtain a precipitant solution. In a water bath at 60°C, add the metal ion solution and the precipitant solution dropwise under stirring for co-precipitation. After the dropwise addition was completed, the mixture was aged for 1 hour. Wash the precipitate to remove impurity ions, then add 1.68g Al(OH) 3 . The mixture thus obtained was added with 150ml of deionized water, heated to 60°C and stirred for 30min. The filter cake obtained by suction filtration was dried at 120° C. overnight, and the temperature was raised to 450° C. in a muffle furnace at 10 K / min and calcined for 4 hours to obtain catalyst A.
Embodiment 2
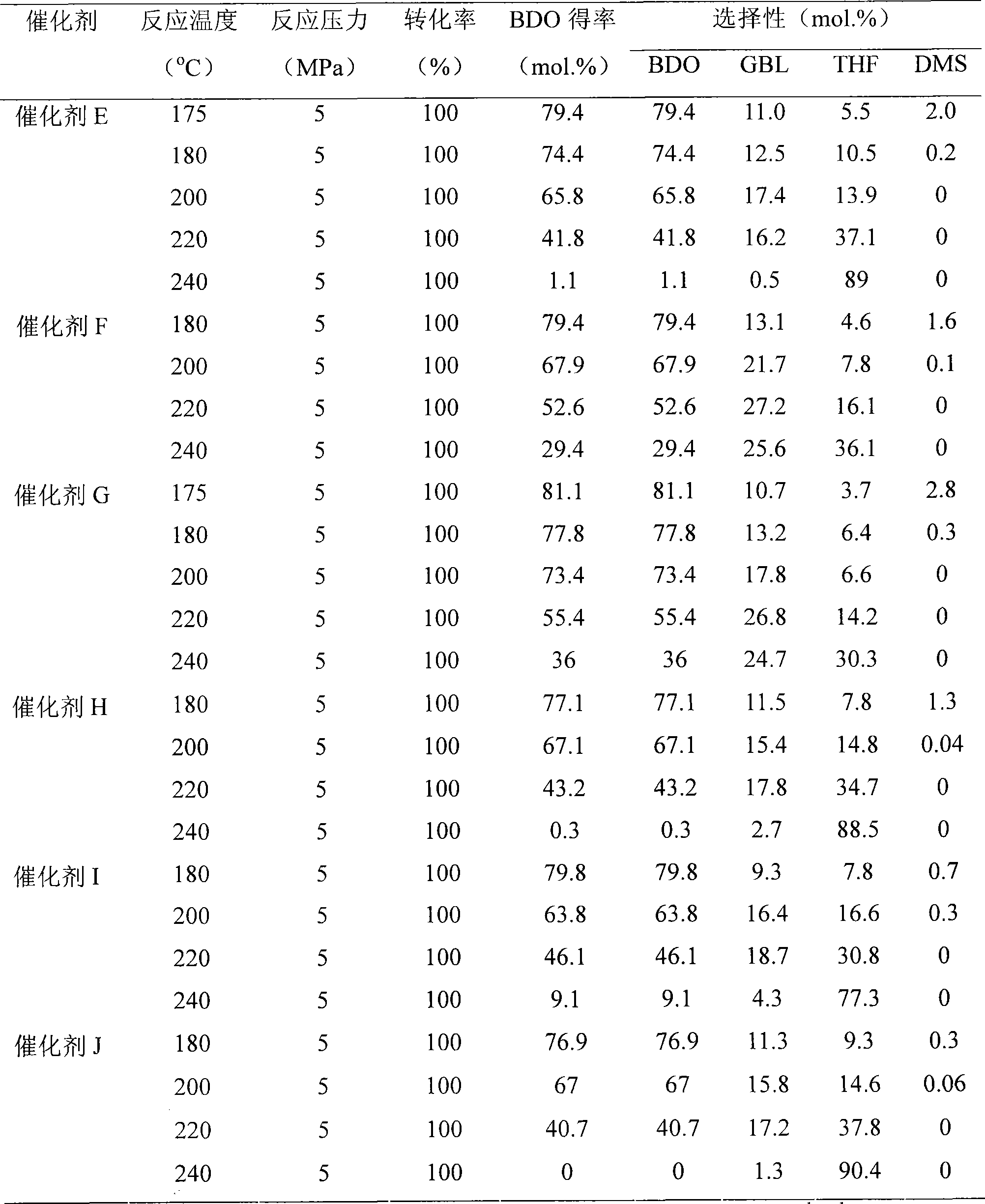
[0022] 10.0g Cu(NO 3 ) 2 ·3H 2 O, 9.8g Zn(NO 3 ) 2 ·6H 2 O and 0.15g Mn(NO 3 ) 2 Dissolve in 75ml H 2 O is made into metal ion solution, 8.7g Na 2 CO 3 Dissolved in 82ml H 2 O to obtain a precipitant solution. In a water bath at 60°C, add the metal ion solution and the precipitant solution dropwise under stirring for co-precipitation. After the dropwise addition was completed, the mixture was aged for 1 hour. Wash the precipitate to remove impurity ions, then add 1.68g Al(OH) 3 . The mixture thus obtained was added with 150ml of deionized water, heated to 60°C and stirred for 30min. The filter cake obtained by suction filtration was dried at 120° C. overnight, and the temperature was raised to 450° C. in a muffle furnace at 10 K / min for 4 hours to obtain catalyst B with a Mn content of 1%. Change the added Mn(NO 3 ) 2 Using the same conditions to prepare catalysts C and D with Mn contents of 2% and 5%, respectively.
Embodiment 3
[0024] 10.0g Cu(NO 3 ) 2 ·3H 2 O, 9.8g Zn(NO 3 ) 2 ·6H 2 O and 0.21g Mg(NO 3 ) 2 ·6H 2 O dissolved in 75ml H 2 O is made into metal ion solution, 8.7g Na 2 CO 3 Dissolved in 82ml H 2 O to obtain a precipitant solution. In a water bath at 60°C, the metal ion solution and the precipitant are added dropwise under stirring for co-precipitation. After the dropwise addition was completed, the mixture was aged for 1 hour. Wash the precipitate to remove impurity ions, then add 1.68g Al(OH) 3 . The mixture thus obtained was added with 150ml of deionized water, heated to 60°C and stirred for 30min. The filter cake obtained by suction filtration was dried overnight at 120° C., and the temperature was raised to 450° C. in a muffle furnace at 10 K / min for 4 hours to obtain catalyst E with a Mg content of 1%. Change the added Mg(NO3 ) 2 Using the same conditions to prepare catalysts F and G with Mg contents of 2% and 5%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com