Enzymatic resolution method of dl 1-phenylethanol compounds
A technology for phenylethanol and compounds, which is applied in the field of enzymatic splitting of racemic 1-phenylethanol compounds, can solve the problems of low yield, unsuitable for large-scale production, etc., and achieves environmental friendliness and great implementation value. and social benefits, the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
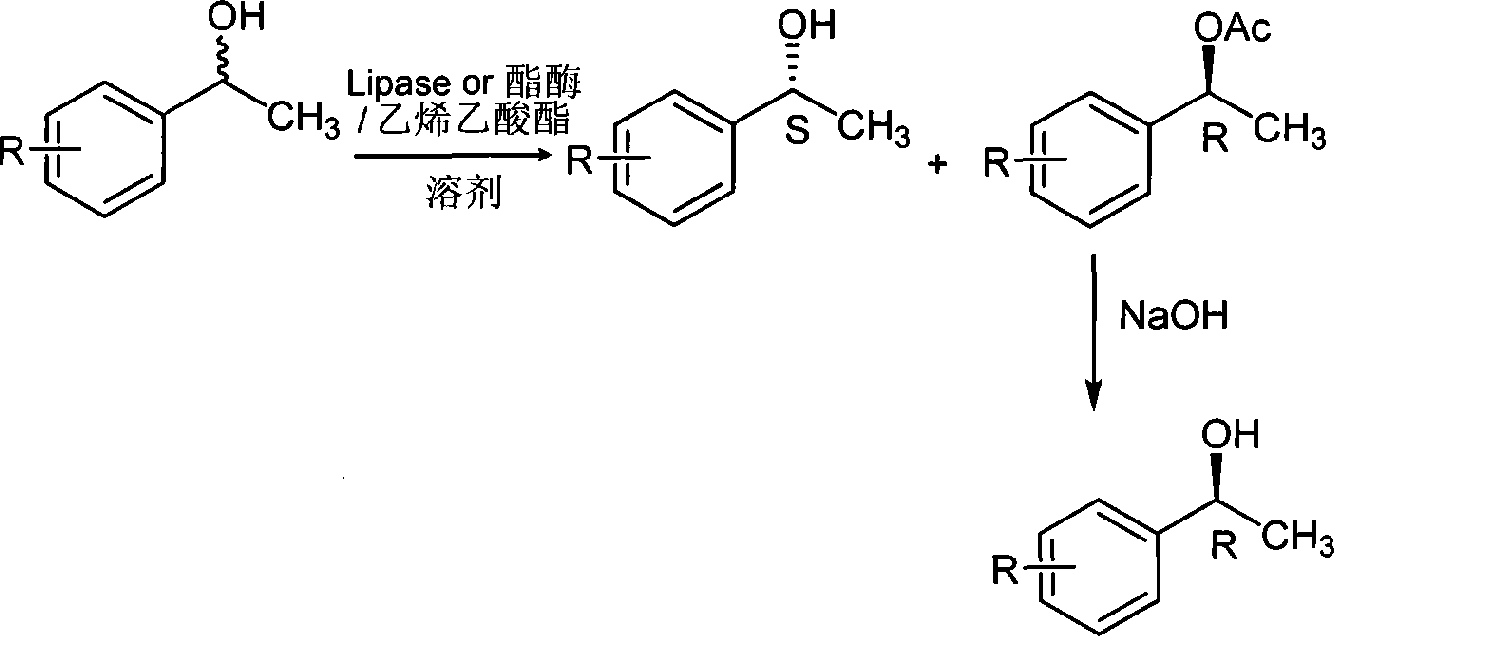
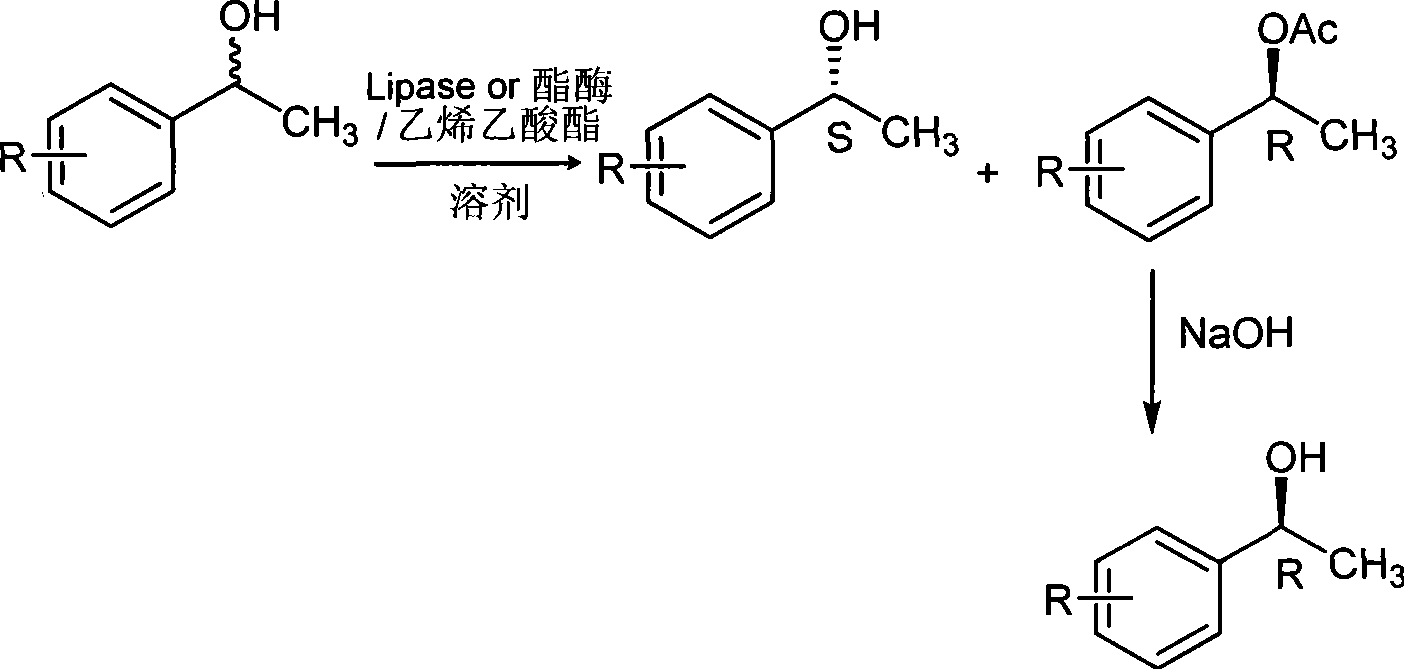
[0127] In the reactor, it is 24.4 (0.02mol) g, 0.5g of biological enzyme, 51.6g (0.6mol) of vinyl acetate and 50ml of solvent cyclohexane to drop into the racemic 1-phenylethanol feeding amount successively, and the enzyme used is lipase AYS "Amano".
[0128] In a 250ml three-necked flask equipped with a thermometer, drying tube, and magnetic stirring, add 24.4g (0.2mol) of 1-phenylethyl alcohol, 8.6g (0.1mol) of vinyl acetate, 50ml of cyclohexane, and 0.5g of biological enzyme, After the addition, the temperature was fixed at 30°C, and the reaction was carried out for 5 hours. During the period, TLC plate monitoring or gas chromatography monitoring was completed. After the reaction was completed, the organic solvent and unreacted vinyl acetate were evaporated, and the residual liquid was separated to obtain colorless R- 1-phenylethyl alcohol acetate liquid and colorless S-(+)-1-phenylethyl alcohol liquid.
[0129] R-1-phenylethanol acetate was hydrolyzed with 5 mol / L NaOH so...
Embodiment 2
[0132] The feed amount of racemic 1-phenylethanol is 24.4g (0.2mol), biological enzyme 0.5g, vinyl acetate 86g (1mol) into the reactor successively, and the enzyme used is esterase XL23.
[0133] After the addition was complete, the temperature was fixed at 30° C., monitored by TLC plate or gas chromatography during the period, and the reaction time was 36 hours.
[0134] Other operations were the same as in Example 1 to obtain colorless S-(-)-1-phenylethanol and R-(+)-1-phenylethanol.
[0135] S-(-)-1-phenylethanol, yield 26%, ee value 96%; R-(+)-1-phenylethanol, yield 34%, ee value 98%. The boiling point is 87-89°C / 10mm Hg, and the melting point is 8-11°C.
Embodiment 3
[0137] The amount of feeding of racemic 1-phenylethanol is 24.4g (0.2mol) into the reactor successively, the biological enzyme used is 0.5g, vinyl acetate 172g (2mol), the used enzyme is esterase XL23 and esterase XL6 and the mass ratio of the two is 1:3.
[0138] After the addition was complete, the temperature was fixed at 40° C., monitored by TLC plate or gas chromatography during the period, and the reaction time was 72 hours.
[0139] Other operations were the same as in Example 1 to obtain colorless S-(-)-1-phenylethanol and R-(+)-1-phenylethanol.
[0140] S-(-)-1-phenylethanol, yield 46%, ee value 98%; R-(+)-1-phenylethanol, yield 49%, ee value 99%. The boiling point is 87-89°C / 10mm Hg, and the melting point is 8-11°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com