New polyether alcohols containing alkoxysilyl groups and method for production
一种烷氧基甲硅烷基、化烷氧基硅烷的技术,应用在化学仪器和方法、有机化学、周期表第4/14族元素的化合物等方向,能够解决不能适用单-和/或多重改性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
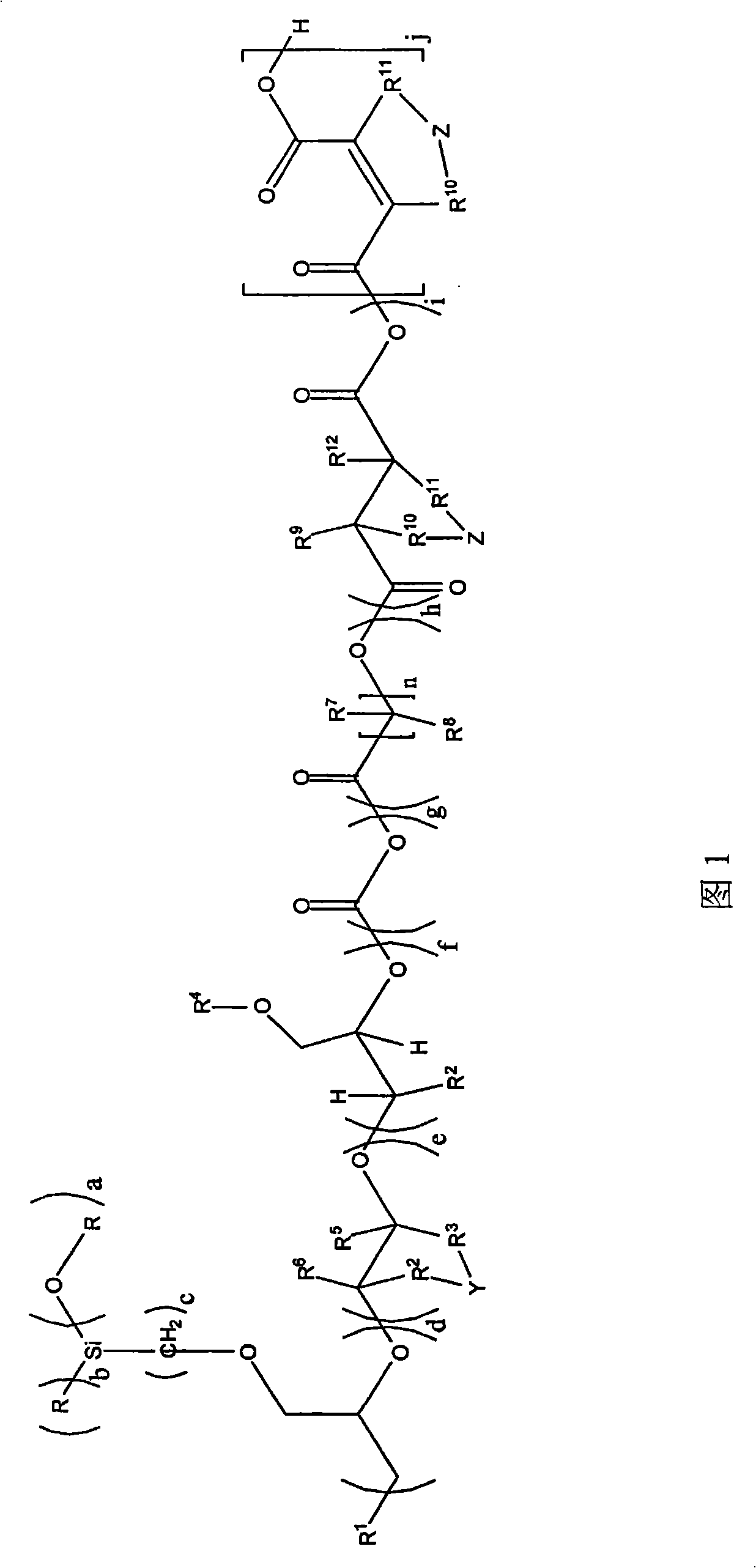
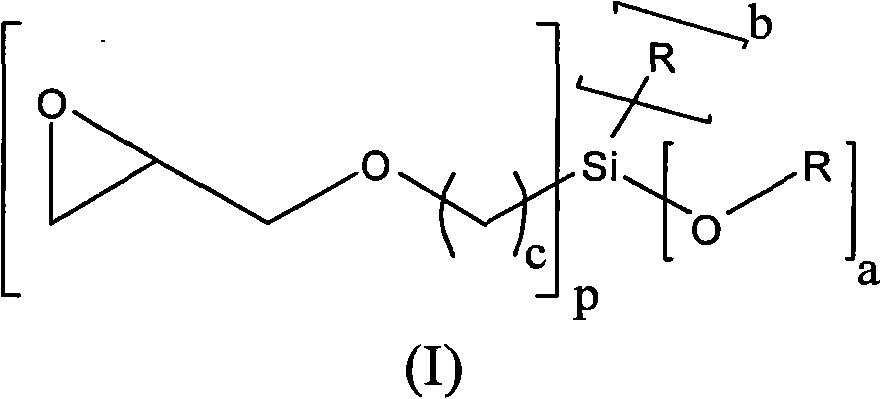
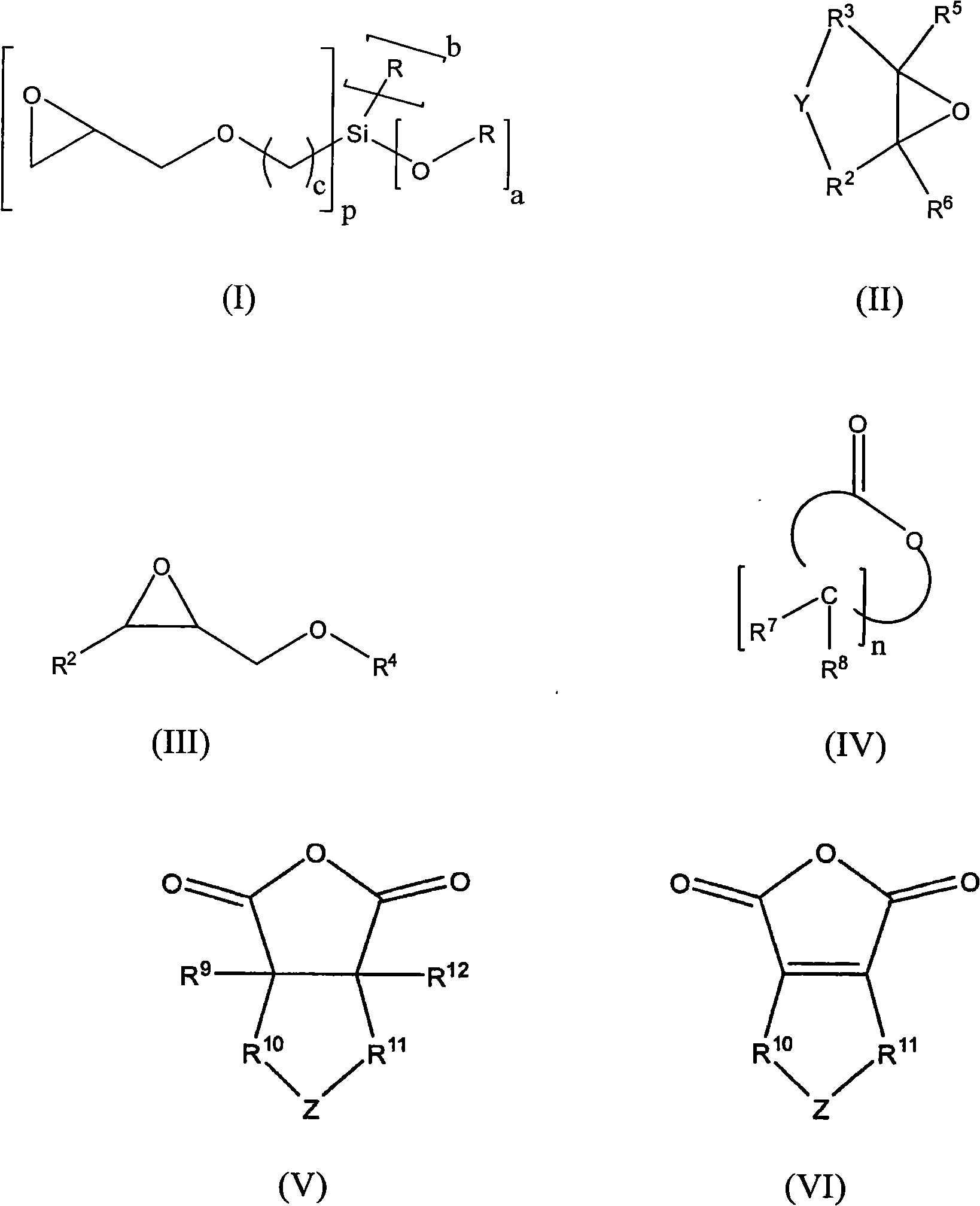
[0138] First, under nitrogen, put 130.2g of polypropylene glycol monobutyl ether (average molar mass of 520g / mol) and 0.10g of zinc hexacyanocobaltate DMC catalyst into a 3-liter autoclave, and stir and heat to 130°C . The reactor was evacuated to an internal pressure of 30 mbar in order to remove by distillation any volatile components present. To activate the DMC catalyst, a 58.0 g portion of propylene oxide was added. After 15 minutes the reaction started (reduced internal pressure in the reactor), under the condition of cooling to 130°C and the maximum internal pressure of the reactor was 0.9 bar absolute pressure, 556.0 g 3-glycidyloxypropyltriethoxysilane ( GLYEO) and 1363.0 g propylene oxide. After post-reaction at 130-150° C. for 90 minutes, a degassing step was performed. In this step, volatile fractions such as residual propylene oxide are distilled off under reduced pressure. The final low viscosity and colorless polyether is cooled to below 80°C and discharge...
Embodiment 2
[0141] First, under nitrogen, 200.0 g of polypropylene glycol monobutyl ether (average molar mass: 750 g / mol) and 0.015 g of zinc hexacyanocobaltate DMC catalyst were charged into a 3-liter autoclave. The mixture was heated to 130° C. and then any volatile components were removed at 30 mbar. To activate the DMC catalyst, a portion of 225.0 g of 3-glycidyloxypropyltriethoxysilane ( GLYEO). At the beginning of the reaction (slightly exothermic) and After GLYEO has been consumed, first 59.1 g of ethylene oxide are metered in within 10 minutes and then 225.0 g of 3-shrinkle are metered in within 20 minutes with cooling to 130° C. and a maximum pressure inside the reactor of 0.8 bar absolute. Glyceryloxypropyltriethoxysilane ( GLYEO). After post-reaction at 130-150°C for 90 minutes, a degassing step was performed to remove volatile fractions.
[0142] Depend on The final polyether alcohol formed by GLYEO and ethylene oxide blocks has low viscosity, contains an average of ...
Embodiment 3
[0144] First, under nitrogen, 65.1 g of 1-octanol and 0.065 g of zinc hexacyanocobaltate DMC catalyst were charged into a 3-liter autoclave. The mixture was heated to 130° C. and then any volatile components were removed at 400 mbar. To activate the DMC catalyst, a 58.0 g portion of propylene oxide was added. After the start of the reaction (decrease in internal pressure), 236.0 g of 3-glycidyloxypropane were metered in successively at 130° C. within 35 minutes under the condition that the maximum pressure inside the reactor was 1.5 bar absolute pressure and cooled. Trimethoxysilane ( GLYMO) and then, after 30 minutes of afterreaction, 220.0 g of ethylene oxide were metered in within 10 minutes. After post-reaction at 150°C for 90 minutes, a degassing step was performed to remove volatile fractions.
[0145] Depend on The final polyether alcohol formed by GLYMO and ethylene oxide blocks contains an average of 2 trialkoxysilyl units per molecule, an OH number of 49.4 mg K...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar mass | aaaaa | aaaaa |
| molar mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com