Composite solid electrolyte and preparation method thereof
A solid electrolyte and composite metal technology, which is applied in the preparation of alumina/hydroxide, circuits, secondary batteries, etc., can solve problems that have not been reported in the literature, and achieve high lithium ion migration number and good electrochemical stability , the effect of high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
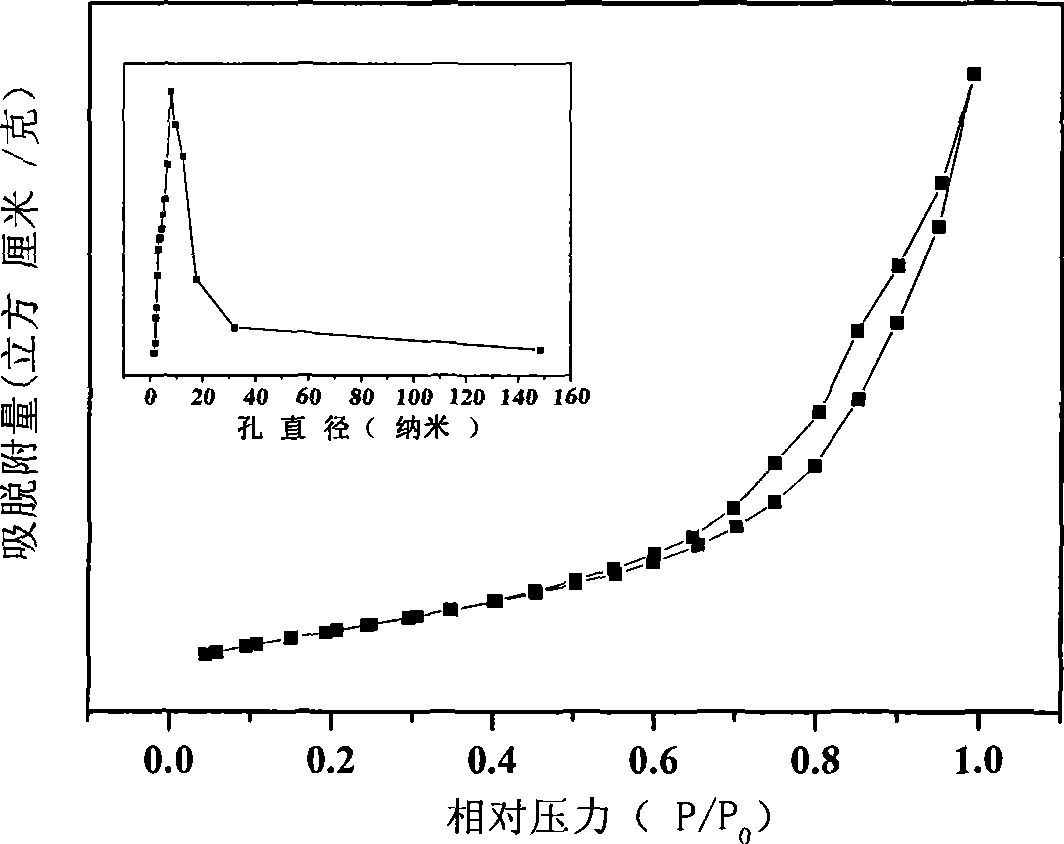
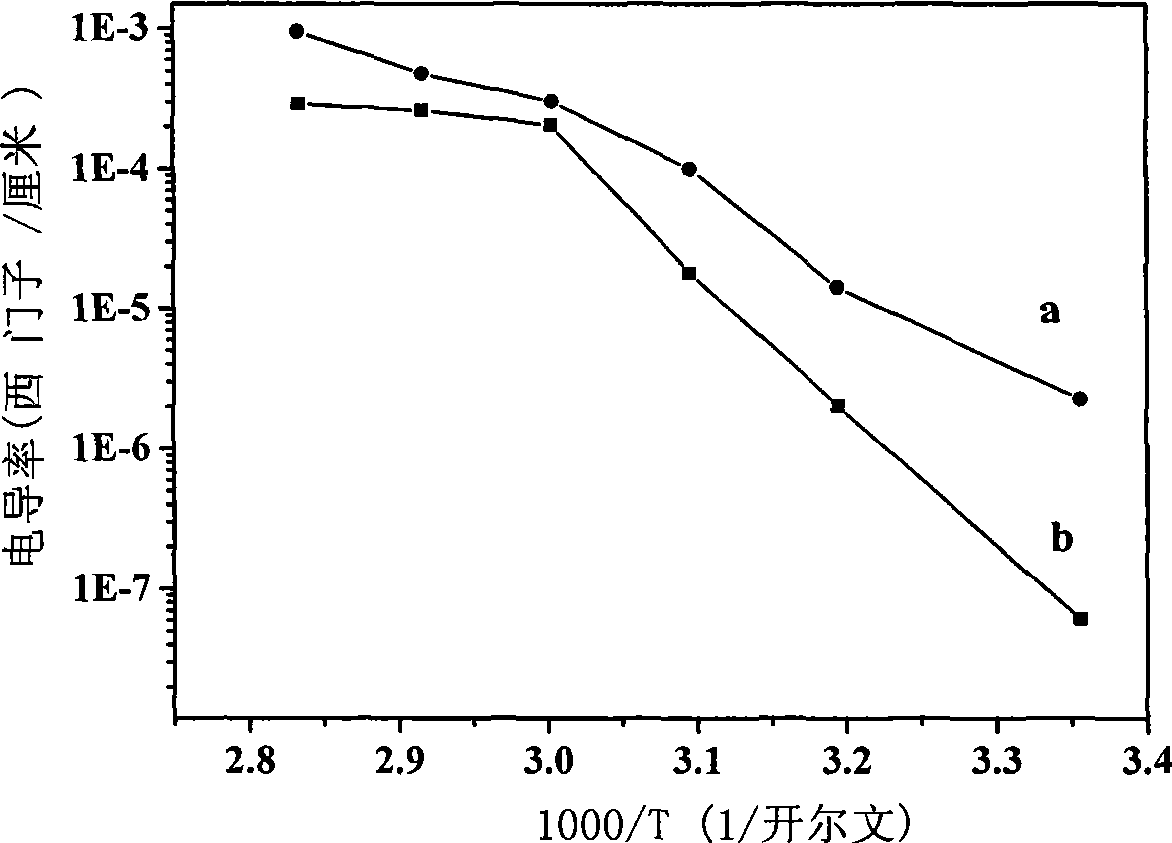
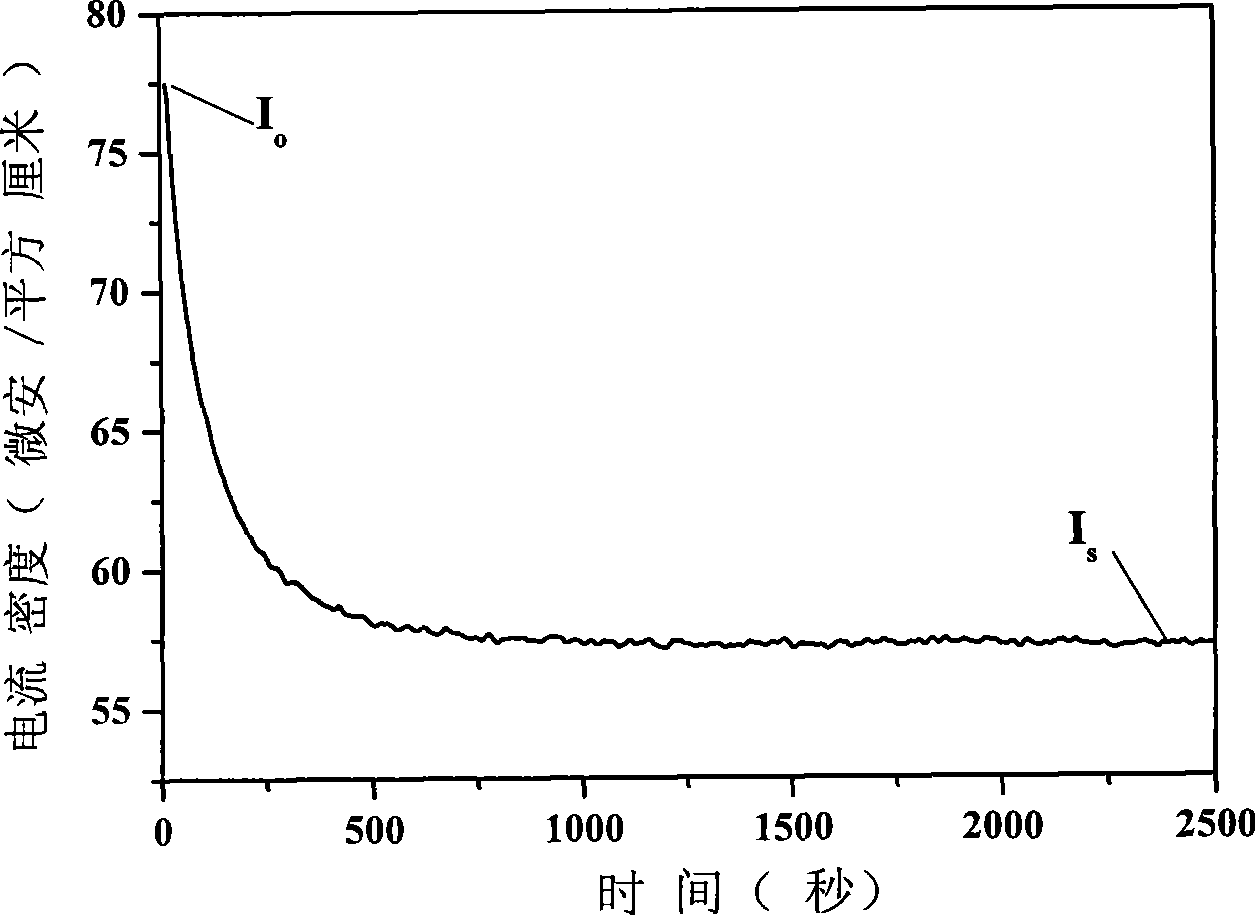
[0027] According to Zn / Al molar ratio is 3 / 1 and the total concentration is [Zn 2+ ]+[Al 3+ ]=1.2mol / L weighs Zn(NO 3 ) 2 ·6H 2 O and Al(NO 3 ) 3 9H 2 O was dissolved in deionized water to prepare a saline solution; according to [CO 3 2- ]=2.0[Al 3+ ], [OH - ]=1.6([Zn 2+ ]+[Al 3+ ]) Weigh NaOH and NaCO 3 Dubbed an alkali solution equal in volume to the salt solution. Pour the above-mentioned salt solution and alkali solution into a colloid mill at the same time, react at a speed of 3000 rpm for 2 minutes, then transfer the white slurry into a three-necked flask for crystallization at 90°C for 4 hours, and suction filter the crystallized product. After washing with deionized water for several times until the pH of the filtrate = 7, the filter cake was oven-dried at 60° C. for 12 hours to obtain a precursor of zinc-aluminum double hydroxy composite metal oxide. Put the above precursor into a muffle furnace, start from room temperature at a rate of 10 °C / min to 700 ...
Embodiment 2
[0030] According to Zn / Al molar ratio is 4 / 1 and the total concentration is [Zn 2+ ]+[Al 3+ ]=1.0mol / L weighs Zn(NO 3 ) 2 ·6H 2 O and Al(NO 3 ) 3 9H 2 O was dissolved in deionized water to prepare a saline solution; according to [CO 3 2- ]=2.0[Al 3+ ], [OH - ]=1.6([Zn 2+ ]+[Al 3+ ]) Weigh NaOH and NaCO 3 Dubbed an alkali solution equal in volume to the salt solution. Pour the above-mentioned salt solution and alkali solution into a colloid mill at the same time, react at a speed of 2000 rpm for 3 minutes, then transfer the white slurry into a three-necked flask for crystallization at 80°C for 5 hours, suction filter the crystallized product, After washing with deionized water for several times until the pH of the filtrate = 7, the filter cake was dried in an oven at 80° C. for 15 hours to obtain a zinc-aluminum double hydroxy compound metal oxide precursor. Put the above precursor into a muffle furnace, start from room temperature at a rate of 12 °C / min to 650 °C...
Embodiment 3
[0033] According to Zn / Al molar ratio is 2 / 1 and the total concentration is [Zn 2+ ]+[Al 3+ ]=1.5mol / L weighs Zn(NO 3 ) 2 ·6H 2 O and Al(NO 3 ) 3 9H 2 O was dissolved in deionized water to prepare a saline solution; according to [CO 3 2- ]=2.0[Al 3+ ], [OH - ]=1.6([Zn 2+ ]+[Al 3+ ]) Weigh NaOH and NaCO 3 Dubbed an alkali solution equal in volume to the salt solution. Pour the above-mentioned salt solution and alkali solution into a colloid mill at the same time, react at a speed of 4000 rpm for 2 minutes, then transfer the white slurry into a three-necked flask and crystallize at 70°C for 6 hours, and suction filter the crystallized product. After washing with deionized water for several times until the pH of the filtrate = 8, the filter cake was oven-dried at 70° C. for 12 hours to obtain a precursor of zinc-aluminum double hydroxy composite metal oxide. Put the above precursor into a muffle furnace, start from room temperature at a rate of 12 °C / min to 750 °C a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com