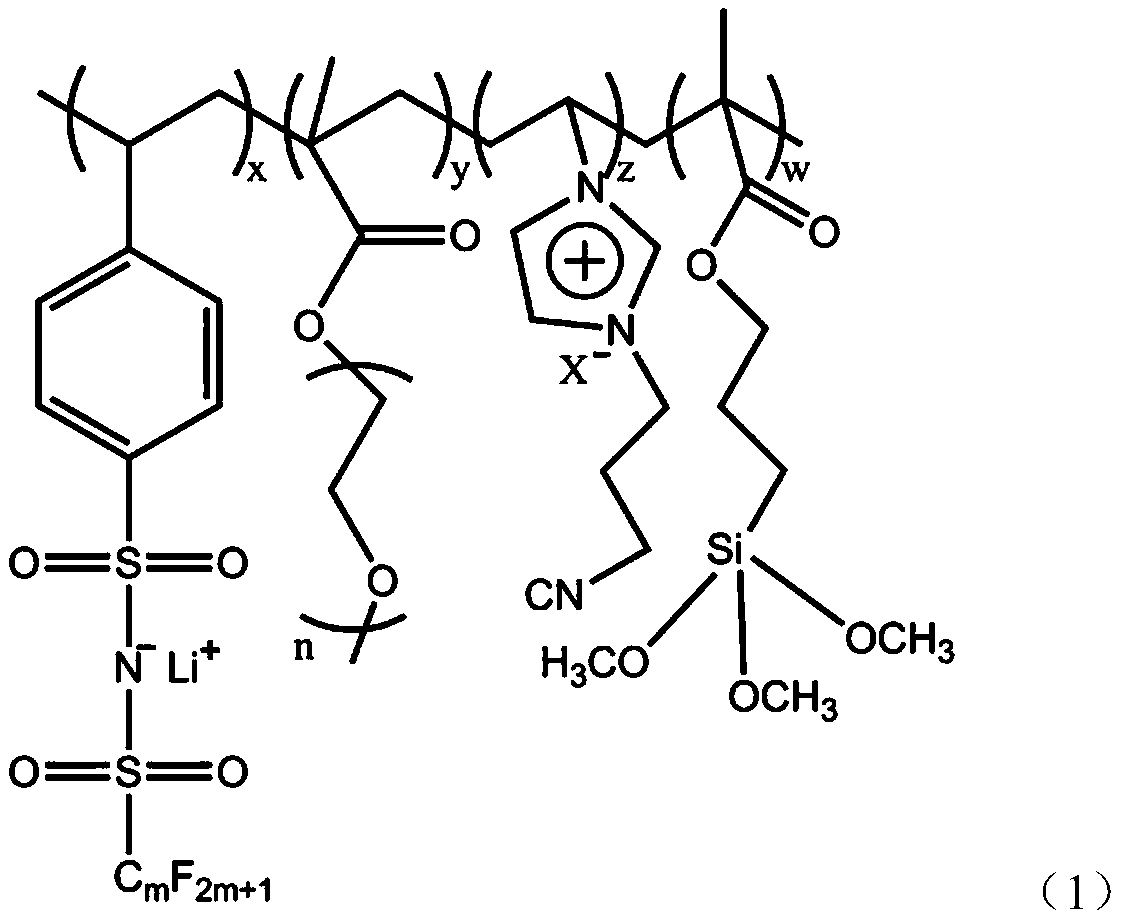
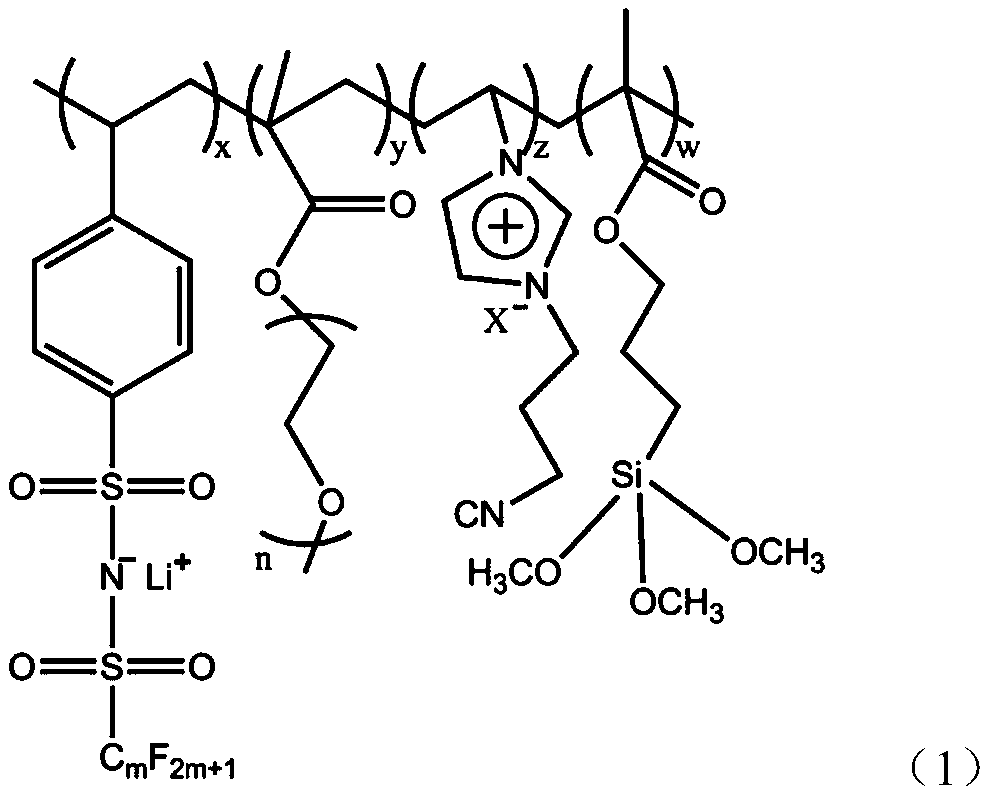
Preparation and application method of composite single-ion solid electrolyte
A solid-state electrolyte and single-ion technology, which is applied in the manufacture of electrolyte batteries, electrolytes, non-aqueous electrolyte batteries, etc., can solve the problems of poor mechanical properties and safety of electrolytes, reduce the mobility of anions, and low ion conductivity, so as to alleviate the concentration difference Polarization issues, excellent thermal and electrochemical stability, effects of high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of single ion polymer electrolyte
[0029] 3.0g lithium p-styrene pentafluoroethylsulfonylimide, 2.0g acrylate polyether, 2.0g cyanoimidazole type ionic liquid, 0.3g silane coupling agent KH-570 and 0.1g photoinitiator 2-hydroxy -2-Methyl-1-phenyl-1-propanone was added to the flask and kept stirring, and it was irradiated with ultraviolet light for 30 minutes. The obtained product was precipitated with dimethyl ether for 3 times, and vacuum After drying, the copolymer of p-styrene pentafluoroethylsulfonimide lithium and acrylic acid polyether is obtained. The structure and composition of the copolymer were characterized by infrared spectroscopy, nuclear magnetic resonance spectroscopy and differential scanning calorimetry (DSC).
Embodiment 2
[0030] Embodiment 2: the preparation of single ion polymer electrolyte
[0031] 6.0g lithium p-styrene pentafluoroethylsulfonimide, 3.0g acrylate polyether, 3.0g cyanoimidazole type ionic liquid, 1.0g silane coupling agent KH-570 and 0.1g photoinitiator 2-hydroxy -2-Methyl-1-phenyl-1-propanone was added to the flask and kept stirring, and it was irradiated with ultraviolet light for 180 minutes. The obtained product was precipitated with dimethyl ether for 3 times, and vacuum After drying, the copolymer of p-styrene perfluorobutylsulfonimide lithium and acrylic acid polyether is obtained. The structure and composition of the copolymer were characterized by infrared spectroscopy, nuclear magnetic resonance spectroscopy and differential scanning calorimetry (DSC).
Embodiment 3
[0032] Embodiment 3: the preparation of single ion polymer electrolyte
[0033] 5.0g lithium p-styrene pentafluoroethylsulfonimide, 2.5g acrylate polyether, 2.5g cyanoimidazole type ionic liquid, 0.6g silane coupling agent KH-570 and 0.1g photoinitiator 2-hydroxy -2-Methyl-1-phenyl-1-propanone was added to the flask and kept stirring, and it was irradiated with ultraviolet light for 90 minutes. The obtained product was precipitated with dimethyl ether for 3 times, and vacuum After drying, the copolymer of p-styrene perfluorobutylsulfonimide lithium and acrylic acid polyether is obtained. The structure and composition of the copolymer were characterized by infrared spectroscopy, nuclear magnetic resonance spectroscopy and differential scanning calorimetry (DSC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com