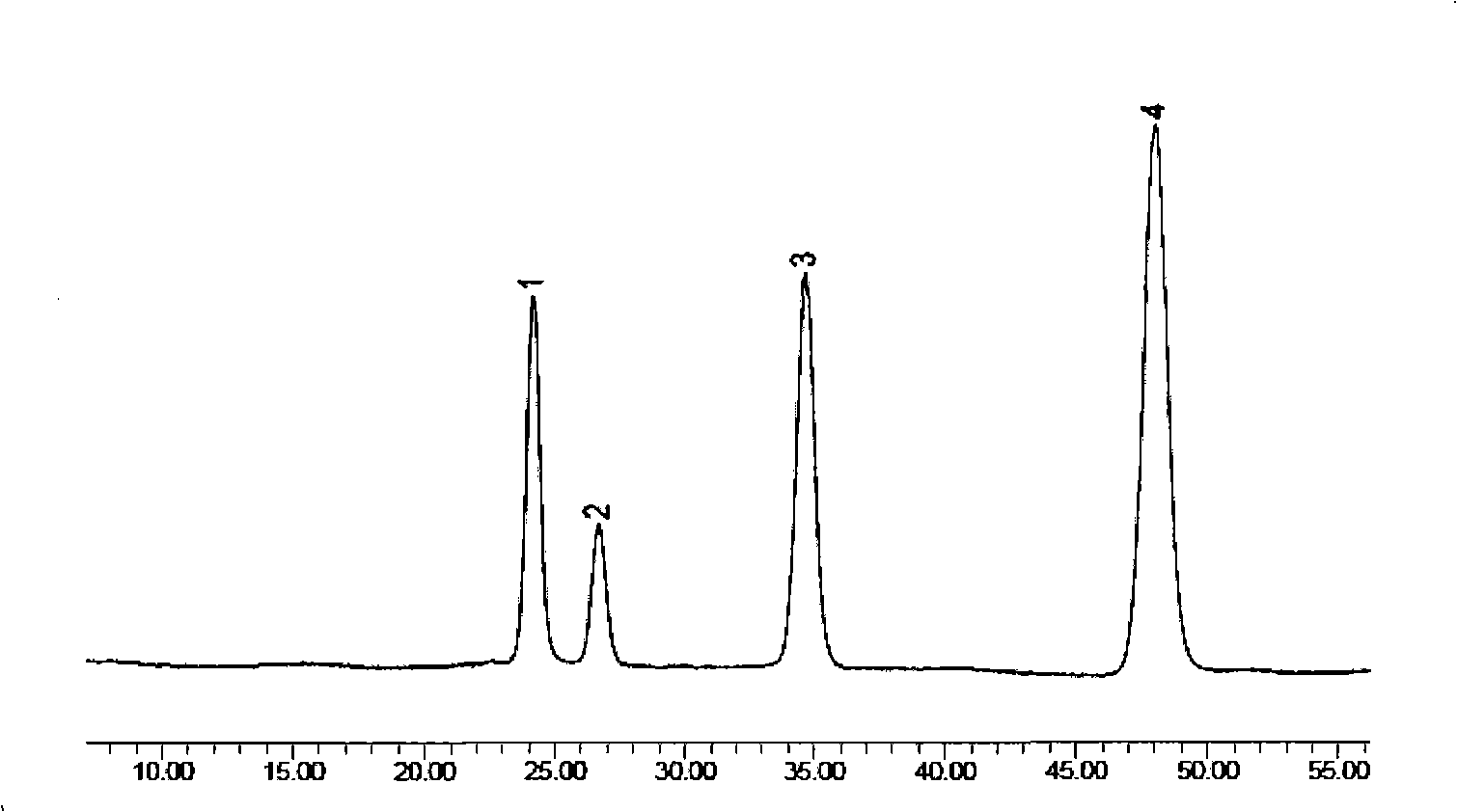
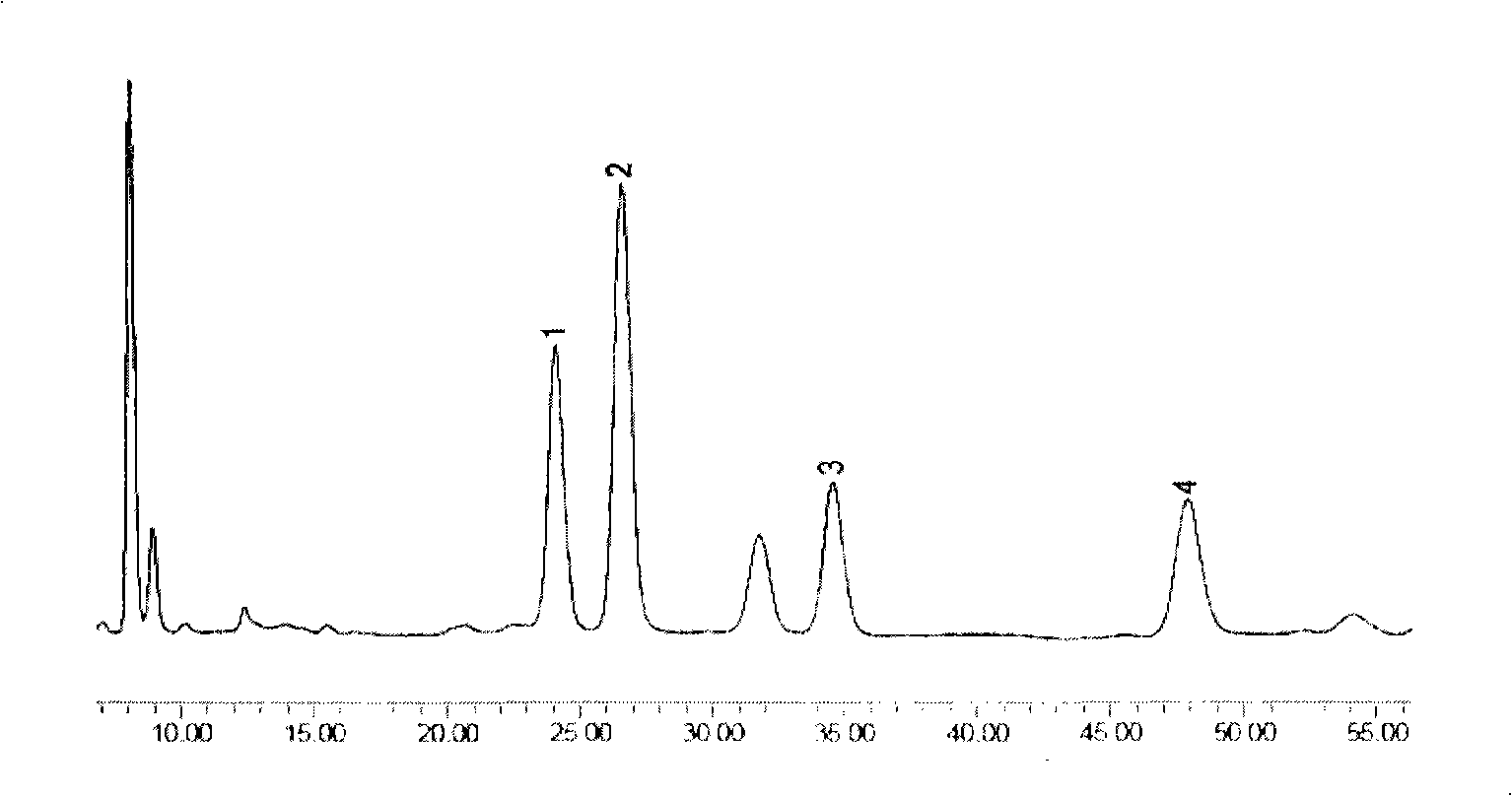
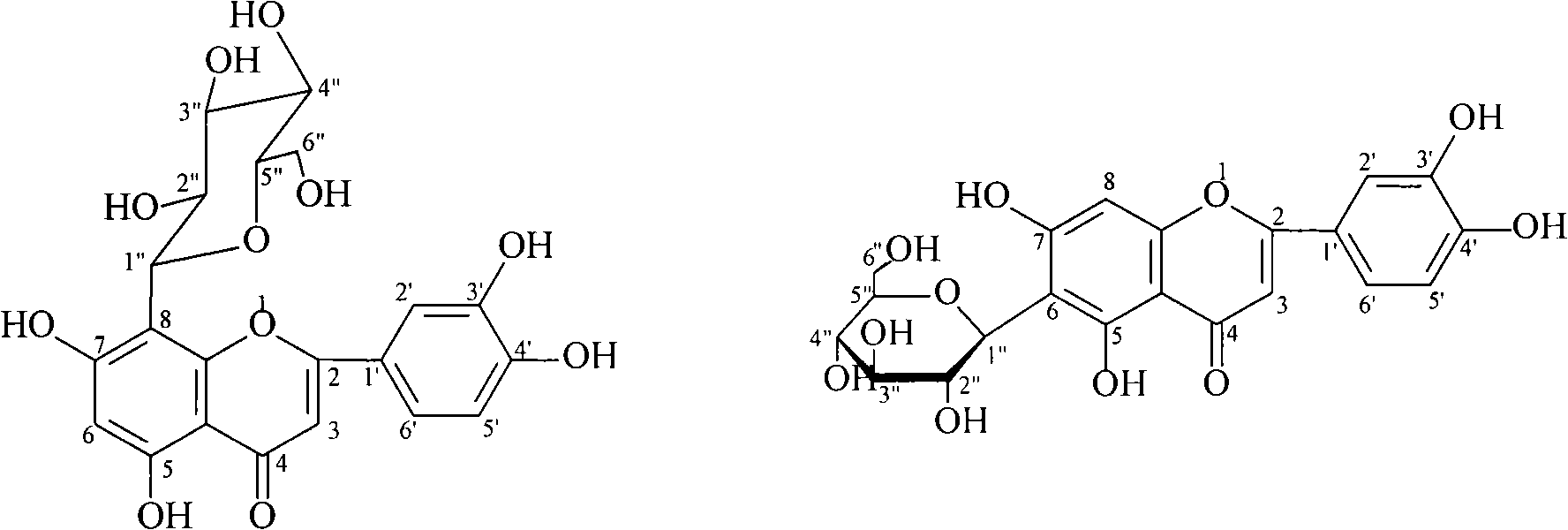
Preparation method of compound bamboo leave flavone dripping pill
A technology of total flavonoids and bamboo leaves, which is applied in the direction of medical preparations containing active ingredients, pill delivery, pharmaceutical formulations, etc., can solve the problems of low dissolution rate and bioavailability, long dissolution time, etc., and achieve easy control of production conditions , fast dissolution time and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Take 7 parts of bamboo leaves and 3 parts of mimosa as raw materials of medicinal materials, mix them and crush them into coarse powder, pass through a 40-mesh sieve, and ultrasonically extract the coarse powder of medicinal materials with 8 times the amount of 60% aqueous ethanol at 50°C each time for 3 times, each time for 45 minutes, suction filtration, and combined filtrates;
[0033]2) Concentrate the filtrate to 1 / 4 of the original volume with a vacuum film at a water bath temperature of 50°C to obtain a concentrated solution, and use powdered activated carbon to sonicate the concentrated solution at 50°C for 25 minutes. The amount of powdered activated carbon is the dry weight of the medicinal material 1.5%, suction filtration, remove activated carbon, continue to concentrate the filtrate to 1 / 10 of the original volume with a vacuum film below 50°C, and obtain the concentrated solution again;
[0034] 3) Extract the concentrated solution obtained again in step...
Embodiment 2
[0039] Step 1) The weight ratio of the raw materials of Chinese medicinal materials is 6 parts of bamboo leaves and 4 parts of Mimosa, ultrasonically extracting with 6 times the amount of 70% aqueous ethanol for 40 min each time; in step 2), the ultrasonic time is 20 min, and the activated carbon is removed by suction filtration , to obtain the filtrate and continue to concentrate to 1 / 8 of the original volume. In step 4), the concentrated paste after n-butanol extraction is dissolved in a small amount of water, passes through the Diaion HP-20 macroporous adsorption resin column, and the diameter-to-height ratio of the chromatographic column is 1: 10. 2 O and 10% aqueous ethanol elution removes water-soluble impurities, then carries out gradient elution with 25% aqueous ethanol, 35% aqueous ethanol, 55% aqueous ethanol successively, and finally all removes all impurities with 70% aqueous ethanol elution, To regenerate the macroporous adsorption resin, the above elution flow ra...
Embodiment 3
[0041] Step 1) The weight ratio of the raw materials of Chinese medicinal materials is 8 parts of bamboo leaves and 2 parts of Mimosa, ultrasonically extracting with 9 times the amount of 65% aqueous ethanol for 50 min each time; in step 2), the ultrasonic time is 28 min, and the activated carbon is removed by suction filtration , to obtain the filtrate and continue to concentrate to 1 / 11 of the original volume. In step 4), the concentrated paste after n-butanol extraction is dissolved in a small amount of water, passes through the Diaion HP-20 macroporous adsorption resin column, and the diameter-to-height ratio of the chromatographic column is 1: 13. 2 O and 10% aqueous ethanol elution removes water-soluble impurities, then carries out gradient elution with 35% aqueous ethanol, 45% aqueous ethanol, 55% aqueous ethanol successively, and finally all removes all impurities with 70% aqueous ethanol elution, To regenerate the macroporous adsorption resin, the above elution flow r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com