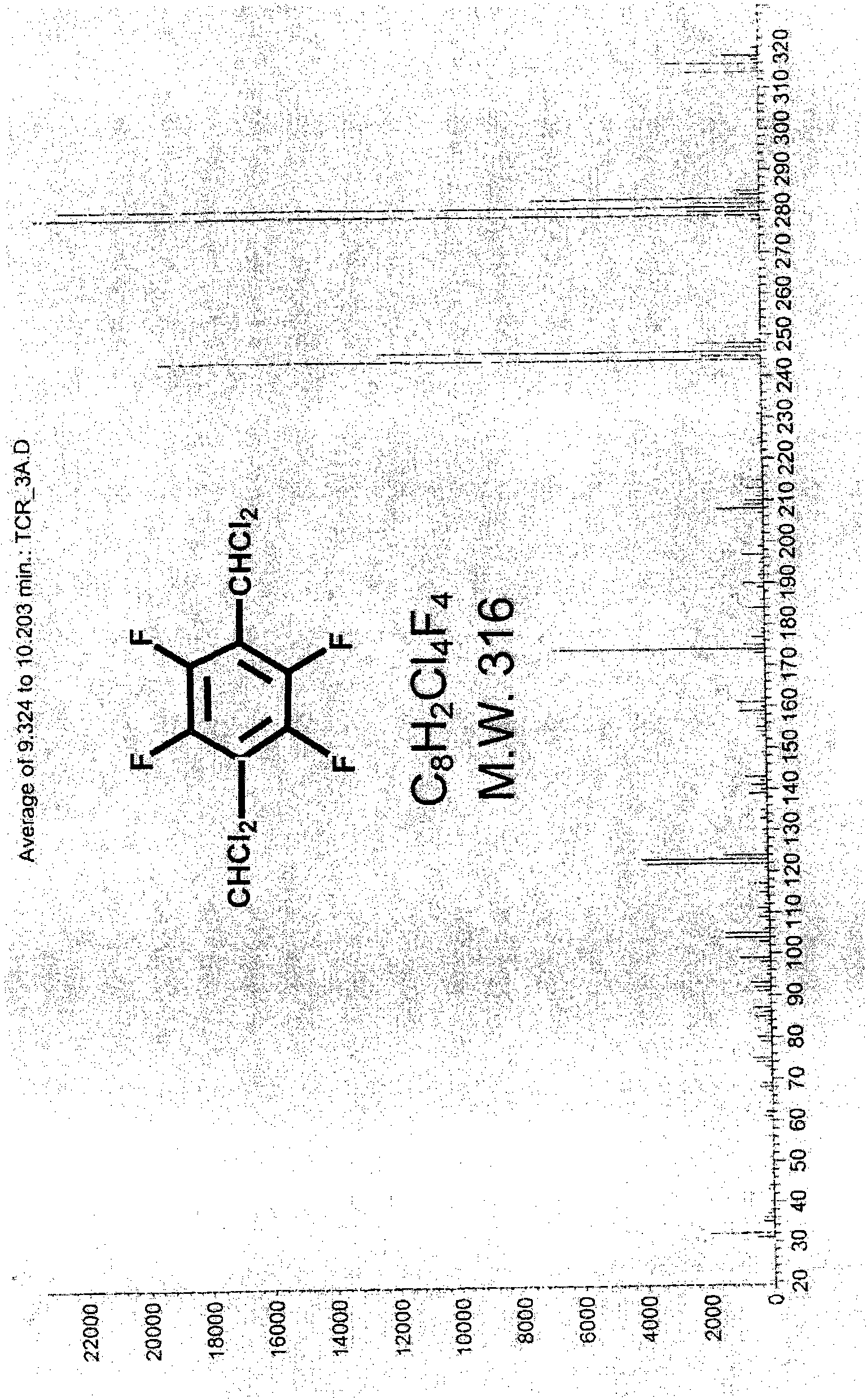
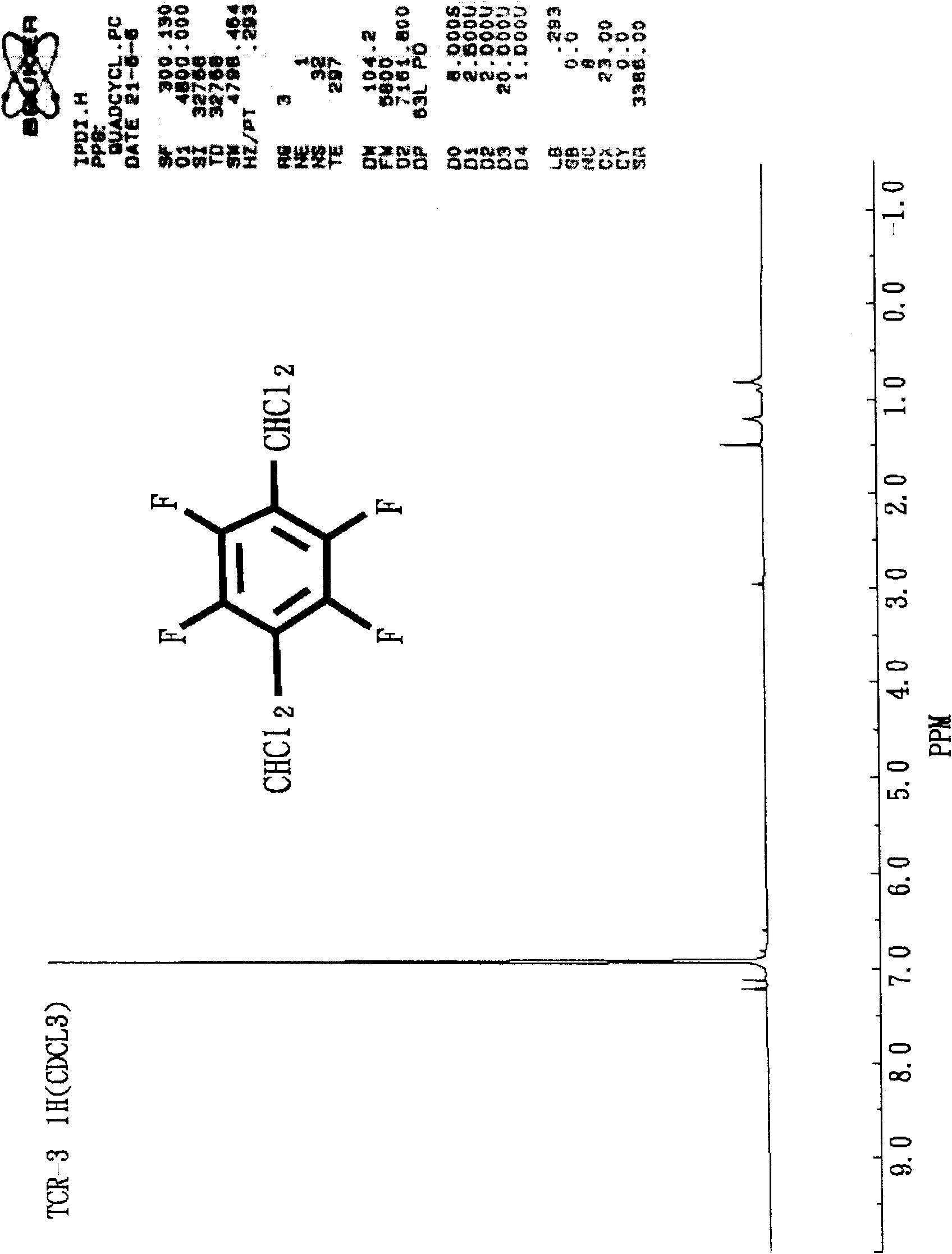
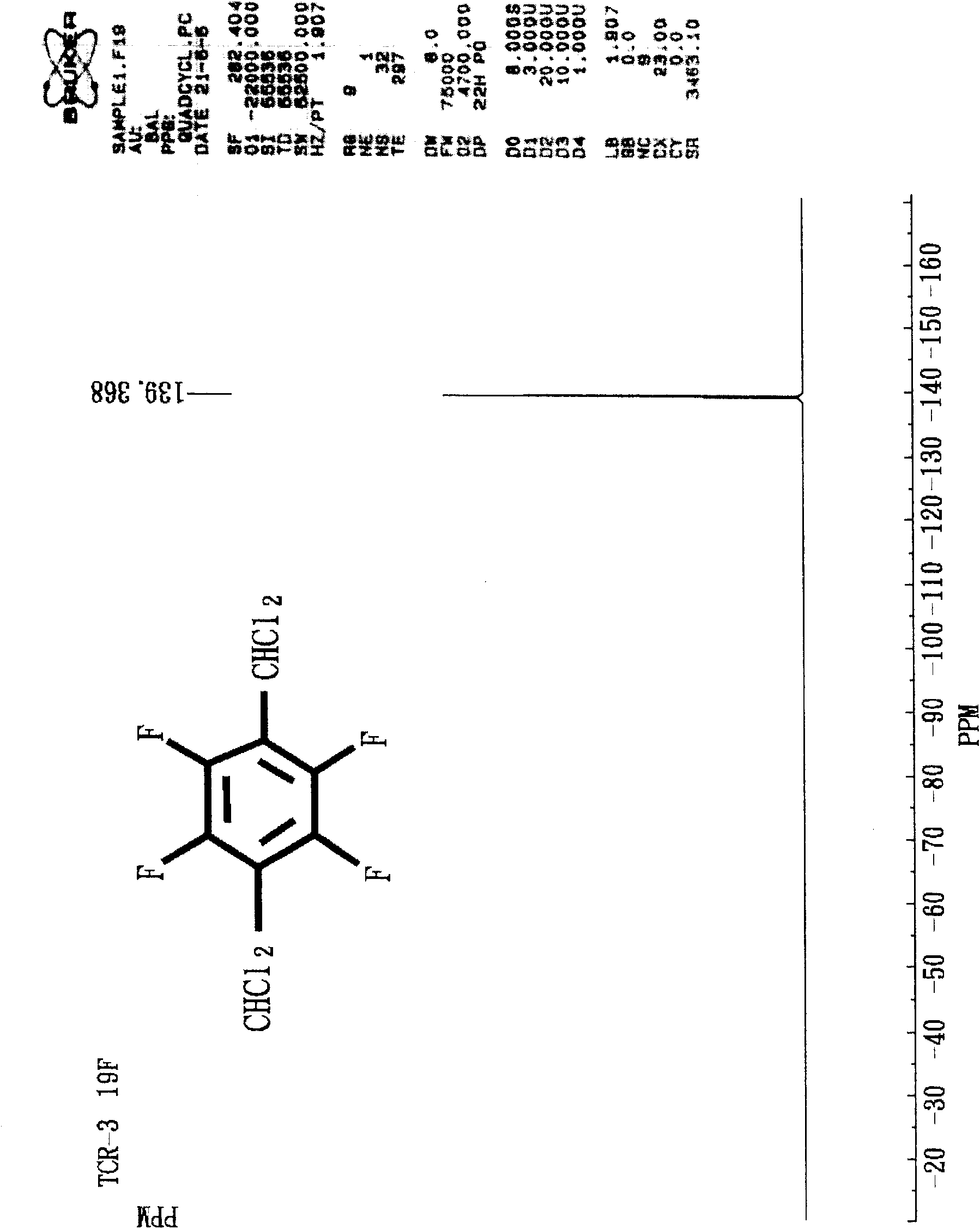
Method for preparing 1,4-bis(dichloromethyl) tetrafluorobenzene
A technology of dichloromethyl and tetrafluorobenzene is applied in the field of preparation of 1,4-bis(dichloromethyl)tetrafluorobenzene, and can solve the problems of long reaction time, low yield, unsuitability for mass production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of 1,4-bis(dichloromethyl)tetrafluorobenzene (with toluene as solvent and dimethylformamide as catalyst)
[0051] Add 15.45gm of tetrafluoroterephthalaldehyde (TFTPA), 3.01gm of dimethylformamide (DMF) and 15.01gm of toluene into a 250ml three-port glass reactor (with temperature probe, condenser tube and ventilation tube) , under nitrogen, slowly add 63.37gm of SOCl from the temperature probe port to the feeding funnel 2 . Then, after replacing the feeding funnel with a temperature probe, start heating with an oil bath and stir with a magnetic stirrer. At this time, the nitrogen can be turned off, and the reflux reaction (85 to 95° C.) is performed for two hours, and gas chromatography (Gas Chromatography; GC) showed that the reaction was complete. After the reaction solution was cooled to room temperature, slowly introduce ice water to remove excess SOCl 2 Hydrolyzed, the water layer was separated after standing for stratification. Then, an ...
Embodiment 2 16
[0057] Embodiment two to sixteen: preparation 1,4-bis(dichloromethyl)tetrafluorobenzene
[0058] The preparation method of embodiment two to sixteen is the same as embodiment one. The amounts of related reactants, solvents, reaction conditions and yields used in Examples 2 to 16 are shown in Table 1.
[0059] Examples two to sixteen show solvent-free or toluene, chloroform, p-xylene, 1,4-dioxane, 1,2-dichloroethane, carbon tetrachloride, tetrahydrofuran, nitrobenzene or O-dichlorobenzene can be used as a solvent for the reaction; the catalyst is the most ideal formamide series compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com