Process for producing cyclic phenol sulfides
A technology of sulfide and phenolic compounds, which is applied in the field of preparation of cyclic phenol sulfides, and can solve problems such as unsuitable for industrialization and less output of cyclic tetramers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
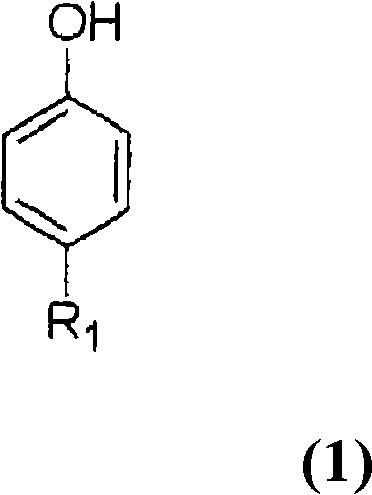
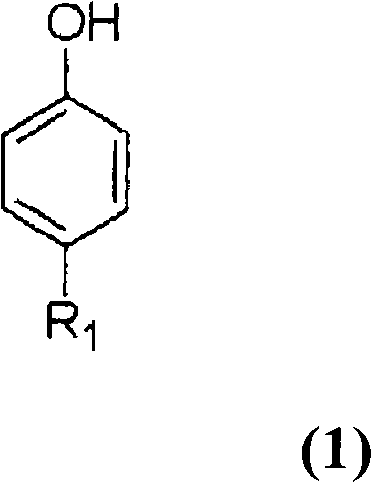
[0042] In a 500ml four-necked flask equipped with a stirrer, a cooling tube, a thermometer and a gas inlet tube, add 45.1 g of 4-tert-butylphenol and 19.2 g of monomeric sulfur (molar ratio to 4-tert-butylphenol: 2 times molar ratio) ) and 6.0 g of sodium hydroxide (molar ratio relative to 4-tert-butylphenol: 0.5 moles), to which 102 g of diphenyl ether was added, and the temperature was raised to 130° C. while stirring in a nitrogen stream, and at 130° C. The reaction was carried out for 1 hour. The temperature was further raised to 170°C, and the reaction was carried out at 170°C for 1 hour. Finally, the temperature was raised to 230°C, and the reaction was carried out at 230°C for 12 hours. During this period, the generated water and hydrogen sulfide were discharged out of the system by flowing nitrogen gas in the reactor, and were removed by contacting and absorbing the aqueous solution of sodium hydroxide, and the reaction was carried out simultaneously. The reaction mi...
Embodiment 2
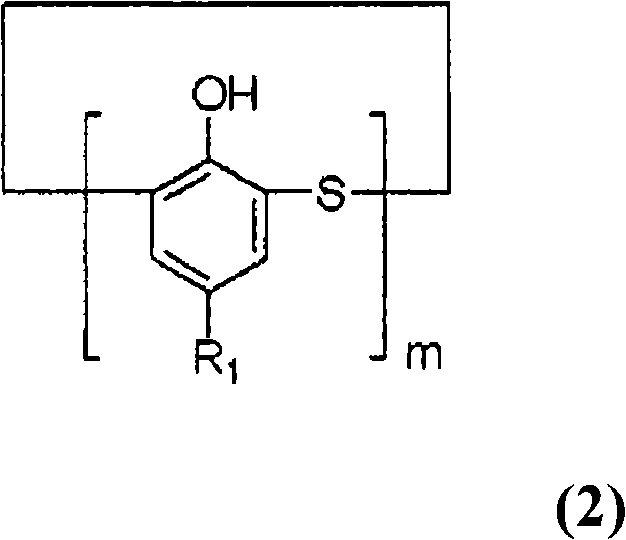
[0044] Under the conditions of Example 1, the cyclization reaction and neutralization with sulfuric acid aqueous solution were also carried out, and 79.1 g of methanol was added to the resulting neutralized reaction mixture, then the temperature was raised to 55 ° C, stirred for 1 hour, and the general formula (2) was precipitated. R1 is a tert-butyl group, and m is a coarse crystal of a cyclic tetramer of 4. The crude crystals obtained by filtering the crude crystals under reduced pressure were washed with 80 g of methanol and then with 80 g of water. The obtained cyclic tetramer was 40.2 g, and the yield was a high yield of 74.4% based on 4-tert-butylphenol. As a result of HPLC analysis, the purity was 98.2% in peak area ratio.
[0045] The structure of the resulting cyclic tetramer was identified by IR measurement. IR (liquid paraffin, nujol) cm -1 : 3243, 1475, 1407, 1393, 1267, 1246, 886, 823, 740.
[0046] Next, the above-mentioned cyclic tetramer was filtered under ...
Embodiment 3
[0049] A cyclic phenol sulfide was prepared in the same manner as in Example 1, except that the molar ratio of monomeric sulfur to 4-tert-butylphenol was changed to 2.5 times molar. As a result, a mixture having a peak area ratio of cyclic tetramer of 95.1% and a peak area ratio of cyclic octamer of 4.1% was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com