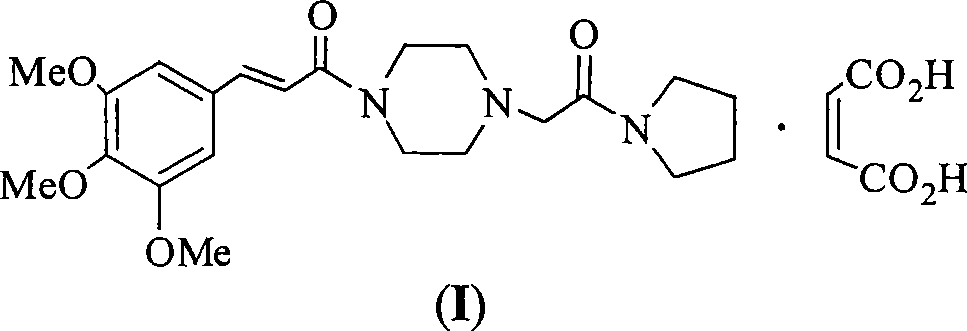
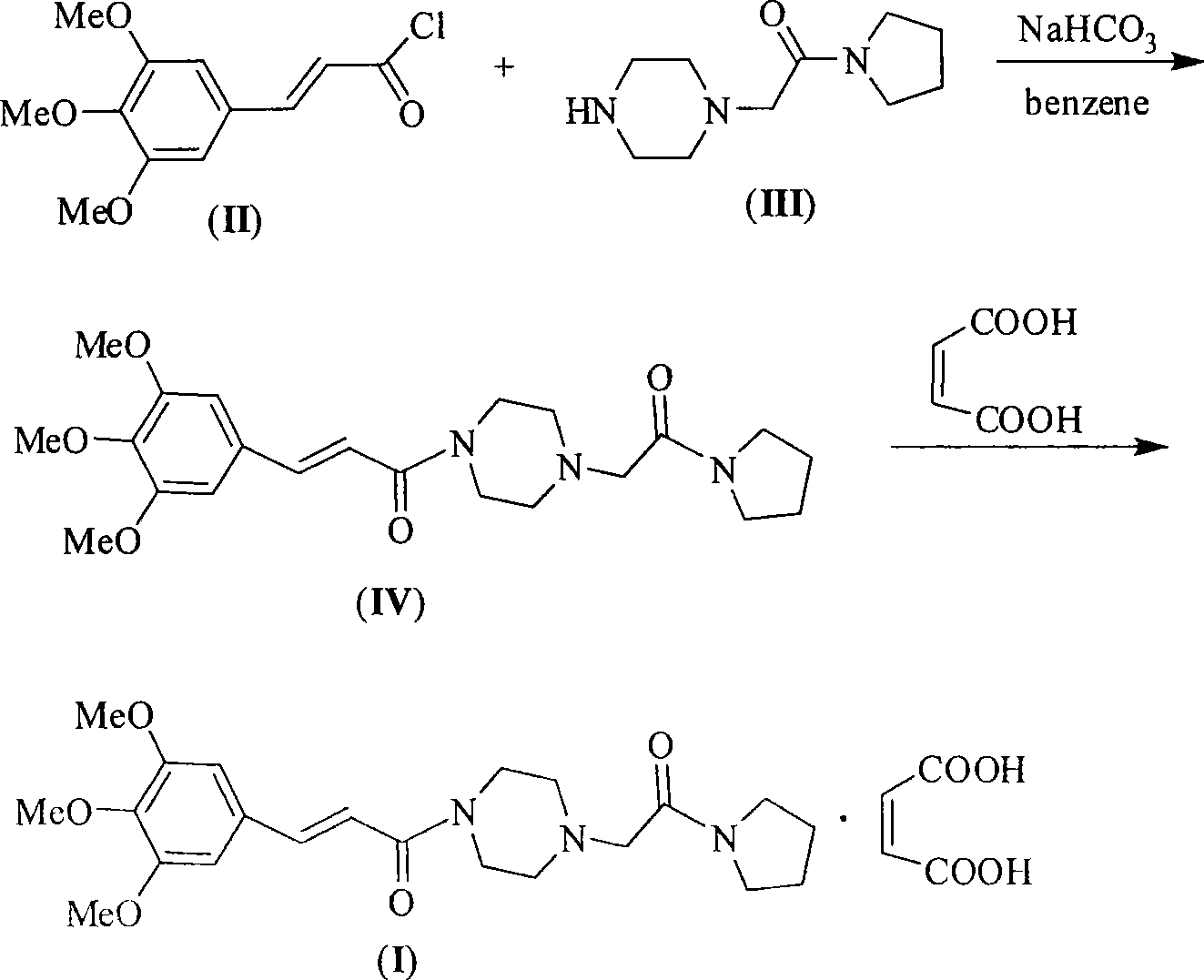
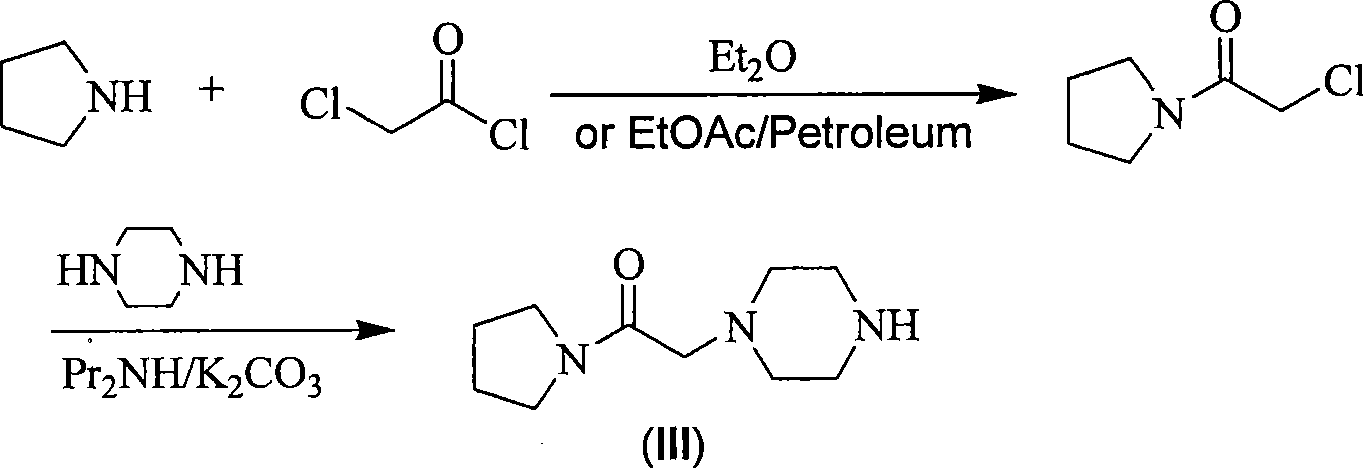
Synthesis method for improved cinepazide maleate
A technology of cinepazide maleate and a synthesis method, which is applied in the synthesis field of cinepazide maleate, can solve the problems of wasting time and energy, cumbersome operation, limited production scale and the like, and achieves avoiding steam distillation and The effect of vacuum distillation and simplified process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1: Preparation of Chloroacetylpyrrolidine
[0034]
[0035] Add 40g of pyrrolidine and 200ml of dichloromethane into a 500ml three-necked flask, cool down to -5°C, and start to drop a total of 250ml of dichloromethane solution dissolved in 63.5g of chloroacetyl chloride and 56.7g of triethylamine. Keep the temperature around 0°C for 2-3 hours to finish dripping. Stir at room temperature for 2 hours, TLC detects that the reaction is complete, stop stirring, filter and wash the filter cake to collect the filtrate, wash the filtrate twice with water, then wash twice with dilute HCl, dry and concentrate the organic phase to obtain chloroacetylpyrrolidine as yellow needle-like crystals 59.5 g, yield: 71.7%.
[0036] Step 2: Preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]-4-tert-butoxycarbonylpiperazine
[0037]
[0038] Add 59.5g of chloroacetylpyrrolidine, 820ml of acetone, 78.4g of mono-tert-butoxycarbonylpiperazine, 66.85g of potassium carbonate, and 7...
Embodiment 2
[0052] The solvent in step 2 of the synthetic method is selected as ethanol, the solvent in step 5 is selected as chloroform, the alkali is selected as sodium hydroxide, and the rest are the same as in Example 1, and the yield is similar to that of Example 1.
Embodiment 3
[0054] The solvent in step 2 of the synthetic method is selected as chloroform, the solvent in step 5 is selected as N,N-dimethylformamide, the base is selected as sodium bicarbonate, and the rest are the same as in Example 1, and the yield is similar to that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com