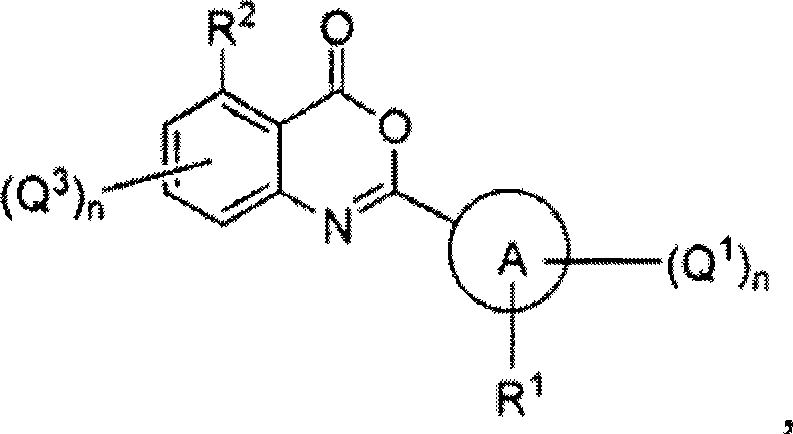
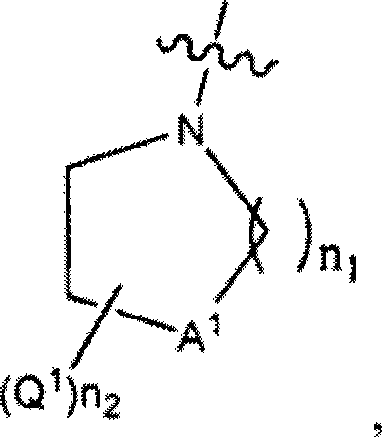
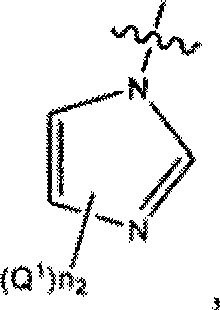
Serine hydrolase inhibitors
A compound and heterocyclic technology, applied in the field of disease compounds, can solve problems such as neutrophil elastase activity cannot be controlled, tissue damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0179] Compound preparation
[0180] The compounds provided by the present invention can be prepared by methods known to those skilled in the art and similar to the following procedures described in the Examples section herein and routine modifications thereof.
[0181] Some exemplary reaction schemes for compound preparation are shown below:
[0182] Process 1:
[0183]
[0184] The leaving group can be any leaving group known to those skilled in the art, such as Br, Cl and F.
[0185] Process 2
[0186]
[0187] Exemplary coupling reagents for the reaction include, but are not limited to: HBTU (2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethylammonium hexafluorophosphate), DCC (N, N'-dicyclohexylcarbodiimide), BOP (benzotriazol-1-yl-oxy-tris-(dimethylammonia)-phosphorus hexafluorophosphate) and others skilled in the art known coupling agents. Any base known to those skilled in the art can be used, exemplary bases are DBU (diazabicyclo[5.4.0]undec-7-ene), DIEA (diisopro...
Embodiment 1
[0317] Example 1. Preparation of 2-(2-imidazol-1-yl-pyridin-3-yl)-5-methyl-4H-benzo[d][1,3]oxazin-4-one (A)
[0318]
[0319]A solution of 2-fluoro-nicotinic acid (2.4 g, 17 mmol) and N,N'-carbonyldiimidazole (CDI, 2.76 g, 17.0 mmol) in anhydrous acetonitrile (12 mL) was stirred at ambient temperature for 30 min, then heated to 65°C for 1 hour. 2-Amino-6-methyl-benzoic acid (2.57 g, 17 mmol) was added to the reaction mixture, followed by stirring at 65°C for 1 hour. Additional CDI (2.76 g, 17 mmol) was added to the stirring reaction mixture and heating was continued to 100 °C for 1 hour. The reaction mixture was then concentrated by rotary evaporator. This crude material was loaded onto a silica gel column. Impurities were removed with a gradient of 0-60% EtOAc / hexanes, and compound 2 was eluted from the column with 100% EtOAc (modified by 5% triethylamine). Rotary evaporation afforded Compound A (2.28 g, 44% yield) as a yellow powder. 1 H-NMR δ(CDCl 3 ): 8.70(dd, 1H,...
Embodiment 2
[0320] Example 2.2-[2-(3-Dimethylamino-pyrrolidin-l-yl)-pyridin-3-yl]-5-methyl-4H-benzo[d][1,3]oxazine- Preparation of 4-keto(46)
[0321]
[0322] Preparation of 2-fluoronicotinic acid esterified Wang resin (B): To a suspension of Wang resin (1.2 mmol / g, 15 g, 18 mmol) in dichloromethane (DCM, 150 mL) was added 2-fluoro-nicotinic acid ( 3.3g, 23.4mmol) and a DC solution (30mL) of 1-hydroxyl 4H-benzotriazole (HOBt, 3.58g, 23.4mmol) and N, N'-dimethylformamide (DMF, 15mL), then add 4 - Dimethylampyridine (DMAP, 286 mg, 2.34 mmol) and N,N'-diisopropylcarbodiimide (DIC, 3.65 g, 23.4 mmol). The mixture was stirred at room temperature for 12 hours. The resin was washed successively with DCM and MeOH, and dried under vacuum to obtain Resin B (17.54 g, 100%).
[0323] Preparation of 2-(3-dimethylamino-pyrrolidin-l-yl)-nicotinic acid (C): To a pyridine suspension (12 mL) of resin B (1.2 mmol / g, 5.0 g, 8.0 mmol) at room temperature Dimethyl-pyrrolidin-3-yl-amine (1.37 mL, 12 mmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com