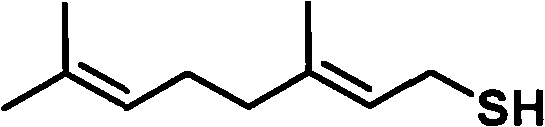
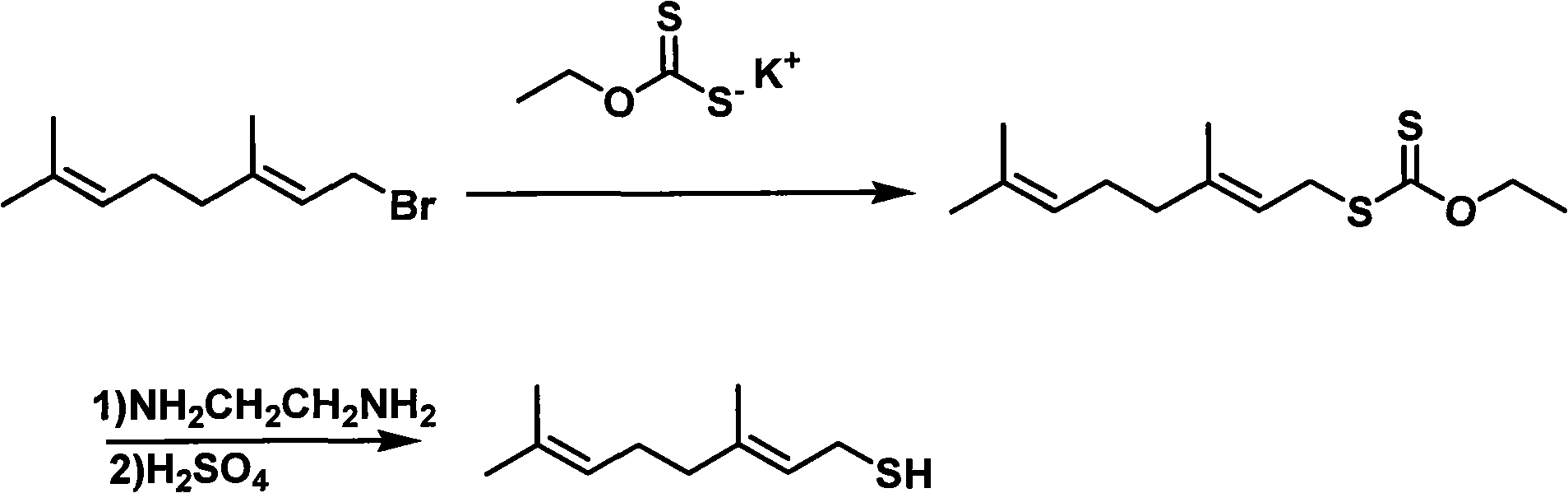
Preparation method of thiogeraniol
A technology for geraniol and geraniol, which is applied in the field of preparation of geraniol, can solve problems such as being unsuitable for industrial-scale production, uneconomical methods, and the like, and achieve the effects of good industrialization prospects, reduced production costs, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of Geranyl Chloride
[0032] At room temperature, 90 milliliters of carbon tetrachloride and 15.42 grams (0.1mol) of geraniol were mixed, and 34.09 grams (0.13mol) of triphenylphosphine were added in four portions within 30 minutes under stirring, and then heated to the reflux of carbon tetrachloride. The temperature was refluxed at 66° C. for 1 hour, and then the reaction system was lowered to room temperature. Add 100 ml of petroleum ether (specification: 30-60 degrees), and continue stirring for 10 minutes. The resulting precipitate was filtered, washed with a small amount of petroleum ether (specification: 30-60 degrees), the filtrate was rotary evaporated to remove the low boiling point solvent, and the residue was rectified under reduced pressure to obtain 6.29 g (37%) of geranyl chloride. The chemical purity measured by gas chromatography was >93%.
[0033] (2) Preparation of geranyl thiol from geranyl chloride
[0034] 22 g (0.128 mol) of gera...
Embodiment 2
[0036] (1) Preparation of Geranyl Bromide
[0037] At room temperature, 36.04 grams (0.11mol) of carbon tetrabromide and 15.42 grams (0.1mol) of geraniol were added to 200 milliliters of dry tetrahydrofuran, and 34.09 grams (0.13mol) of triphenylphosphine were added in four portions within 30 minutes under stirring. Then the reaction system was lowered to room temperature, and after stirring for 1 hour, 400 ml of petroleum ether (specification: 30-60 degrees) was added, and stirring was continued for 10 minutes. The resulting precipitate was filtered, washed with a small amount of petroleum ether (specification: 30-60 degrees), the filtrate was rotary evaporated to remove the low boiling point solvent, and the residue was rectified under reduced pressure to obtain 7.13 g (33%) of geranyl bromide. The chemical purity was >94% as determined by gas chromatography.
[0038] (2) Starting from geranyl bromide to prepare geranyl thiol
[0039] 21.6 g (0.1 mol) of geranyl bromide, 7...
Embodiment 3
[0041] (1) Amplified preparation of geranyl chloride
[0042] At room temperature, 1800 milliliters of carbon tetrachloride and 308.4 grams (2mol) of geraniol were mixed, and 681.8 grams (2.6mol) of triphenylphosphine were added in four portions within 30 minutes under stirring, and then heated to the reflux temperature of carbon tetrachloride Reflux reaction at 66° C. for 1 hour, and then lower the reaction system to room temperature. 2000 ml of n-hexane was added, and stirring was continued for 10 minutes. The resulting precipitate was filtered, washed with a small amount of n-hexane, the filtrate was rotary evaporated to remove the low boiling point solvent, and the residue was rectified under reduced pressure to obtain 179 g (52%) of geranyl chloride. The chemical purity measured by gas chromatography was >93%.
[0043] (2) Scale up the preparation of geranyl thiol from geranyl chloride
[0044] 220 g (1.28 mol) of geranyl chloride, 98 g (1.28 mol) of thiourea and 1000 ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com