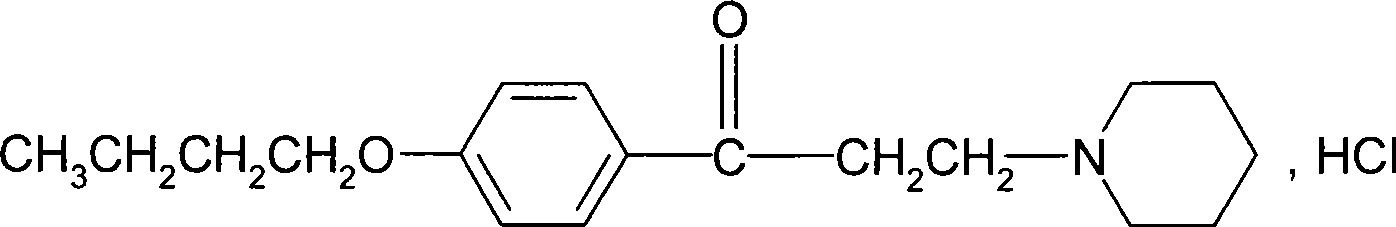
Novel method for synthesizing dyclonine hydrochloride
A technology of dyclonine hydrochloride and a synthesis method, applied in directions such as organic chemistry, to achieve the effects of reducing production costs, being beneficial to industrialized production, and having good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
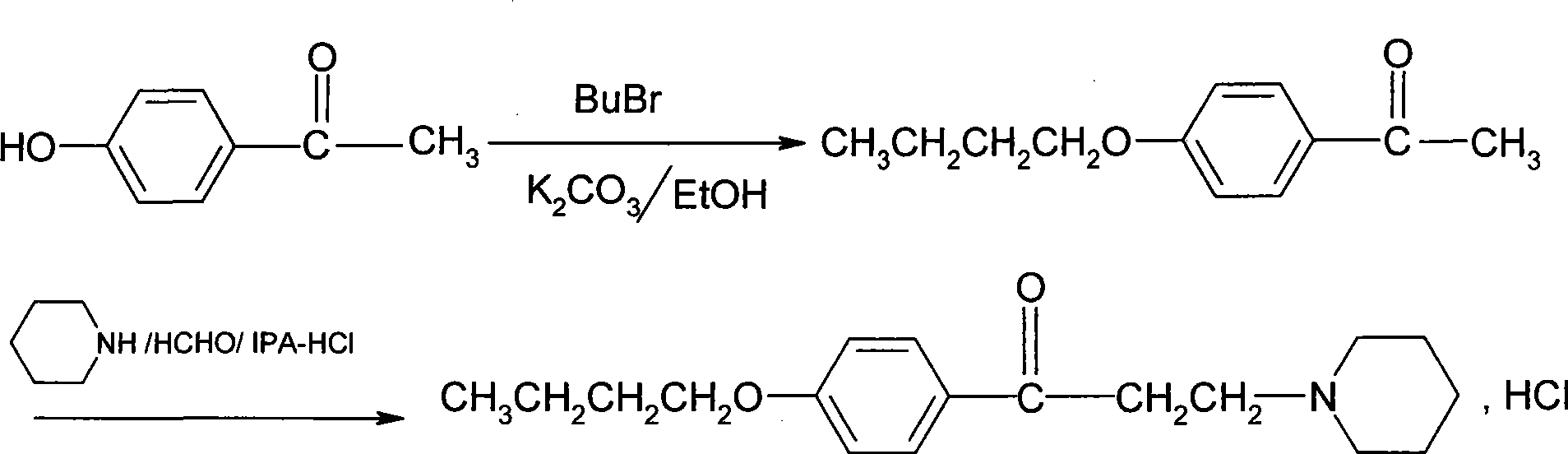
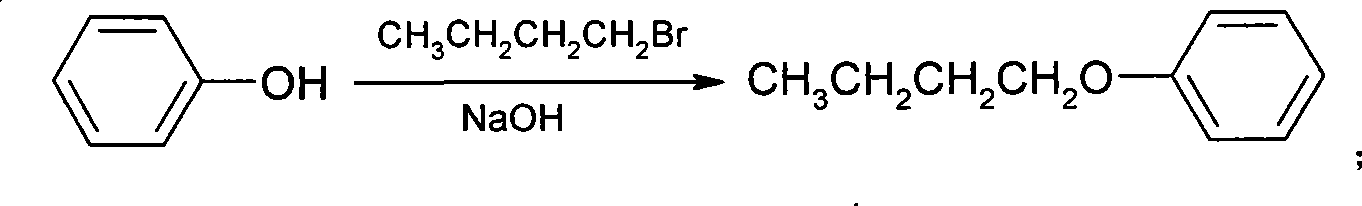
[0049] (1) Synthesis of phenbutyl ether
[0050] Add 48Kg (510mol) of phenol and 84Kg of 30% sodium hydroxide solution into 45Kg of water, add 80Kg of bromobutane (584mol), react at 80°C for 10 hours, let the water layer stand, and wash the oil layer with water until neutral After vacuum distillation, 65.7Kg of phenbutyl ether was obtained with a yield of 85.8% and a content of 98.3%.
[0051] (2) Synthesis of p-butoxyacetophenone
[0052] Add 54Kg (360mol) of phenbutyl ether and 3.6Kg of zinc chloride (28mol) into 54Kg (529mol) of acetic anhydride, reflux for 4 hours, wash with water and sodium carbonate solution, then wash with salt water until neutral, and depressurize After distillation, 58.7Kg of p-butoxyacetophenone was obtained, the yield was 84.9%, and the content was 98.1%.
[0053] (3) Synthesis of Dyclonine Hydrochloride
[0054] Add 40Kg (208mol) p-butoxyacetophenone and 25.6Kg (210mol) piperidine hydrochloride into absolute ethanol, stir, then add 12Kg paraform...
Embodiment 2
[0056] In step (1), the reaction temperature is 100°C, and the weight of phenbutyl ether obtained is 66.3Kg, and the yield is 86.6%. Other steps are with embodiment 1.
Embodiment 3
[0058] In the step (1), 45Kg of phenol and 67.5Kg of water (weight ratio: 1:1.5) were added to obtain 66.7Kg of phenbutyl ether with a yield of 87.0%. Other steps are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com