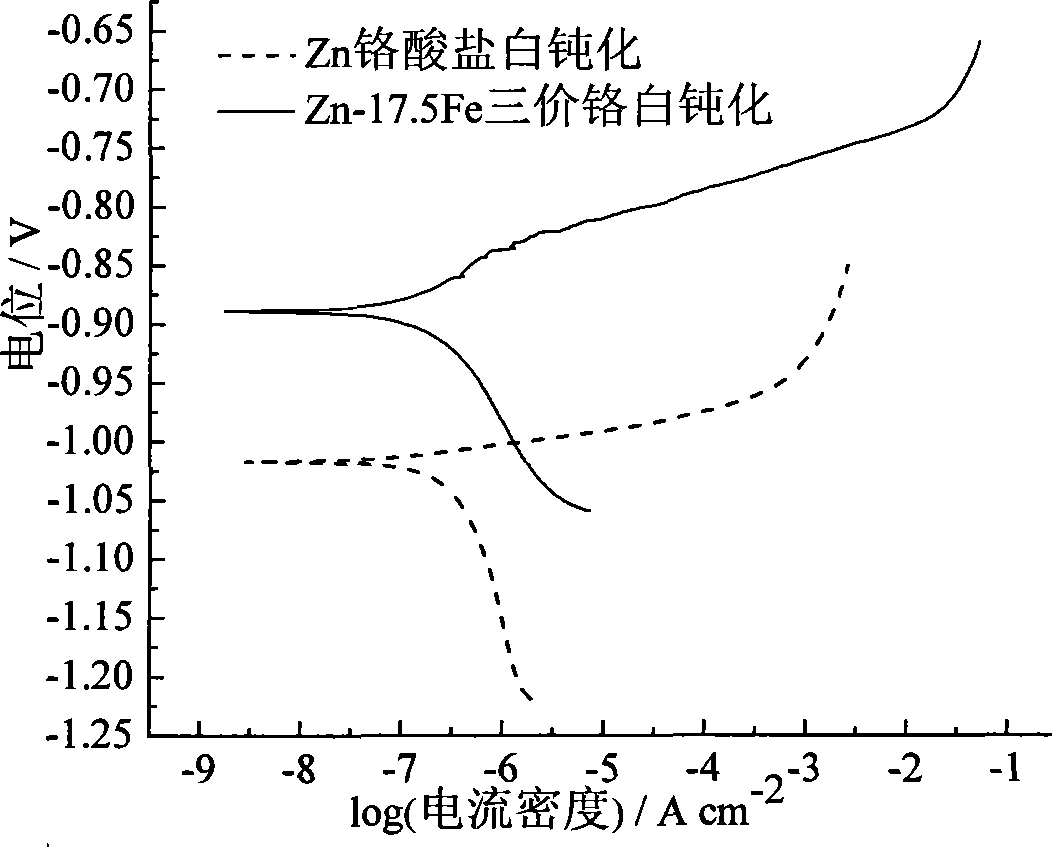
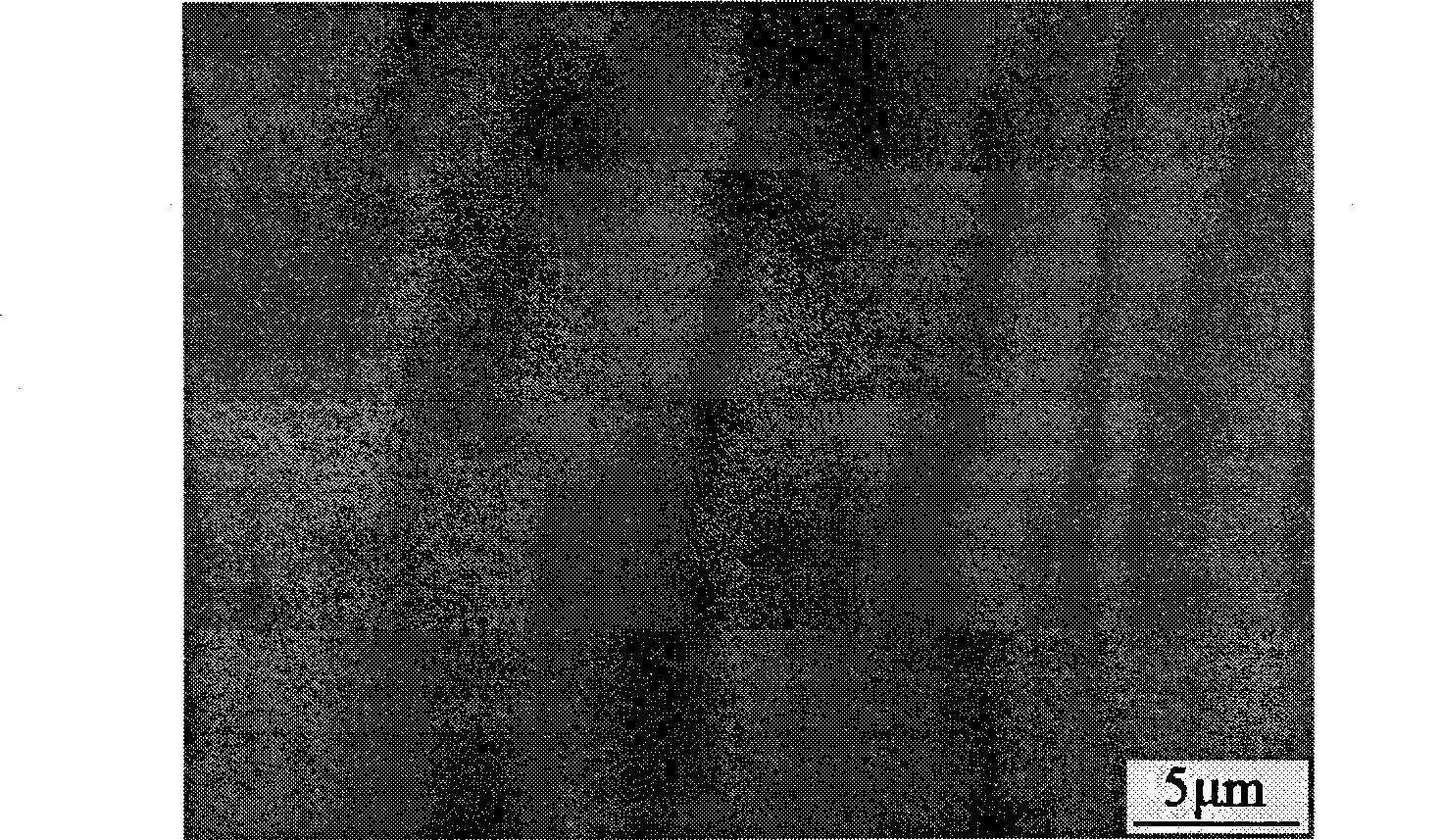Bright corrosion resisting zinc-iron alloy plating process
A zinc-iron alloy and electroplating process technology, which is applied in the field of bright and corrosion-resistant zinc-iron alloy electroplating with alkaline plating solution, can solve the problems of rough coating, low deposition rate, and low equipment pollution, and achieve fine and bright coating, good dispersion ability, and less inclusion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The anode is made of stainless steel with a size of 50mm×40mm×1mm. The cathode is Q235 steel plate with a size of 40mm×30mm×1mm.
[0020] (1) Configure zinc-iron alloy plating solution: prepare 1000mL plating solution, the composition of the plating solution is: ZnO10g, NaOH 130g, triethanolamine 30mL, FeSO 4 ·7H 2 08g, 4mL of the compound (DE) of dimethylamine and epichlorohydrin, 0.8g of ethylenediaminetetraacetic acid, 0.04g of vanillin, 0.02g of sodium dodecylsulfonate, 2g of Zn powder. Add 400mL of water into the plating tank, pour the solid NaOH into the water, stir and dissolve, then add the ZnO that has been made into a paste, and add the NaOH solution under stirring. After it is completely dissolved, add water to 700mL, add 2g of Zn powder, stir for 30 minutes, and then clarify and filter. In a beaker, after diluting triethanolamine with 200 mL of water, FeSO 4 ·7H 2 O was added under stirring, and after it was completely dissolved, it was added to the pla...
Embodiment 2
[0031] The anode is made of stainless steel with a size of 50mm×40mm×1mm. The cathode is Q235 steel plate with a size of 40mm×30mm×1mm.
[0032] (1) Configure zinc-iron alloy plating solution: prepare 1000mL plating solution, the composition of the plating solution is: ZnO 8g, NaOH 100g, triethanolamine 20mL, FeSO 4 ·7H 2 O 7g, compound (DE) of dimethylamine and epichlorohydrin 2mL, ethylenediaminetetraacetic acid 1g, vanillin 0.02g, sodium dodecylsulfonate 0.04g, Zn powder 1g. Add 300mL water into the plating tank, pour the solid NaOH into the water, stir and dissolve, then add the NaOH solution to the ZnO that has been made into a paste while stirring. After it is completely dissolved, add water to 600mL, add 1g of Zn powder, stir for 25 minutes, and then clarify and filter. In a beaker, after diluting triethanolamine with 200 mL of water, FeSO 4 ·7H 2 O was added under stirring, and after it was completely dissolved, it was added to the plating tank, and then the compo...
Embodiment 3
[0036] The anode is made of stainless steel with a size of 50mm×40mm×1mm. The cathode is Q235 steel plate with a size of 40mm×30mm×1mm.
[0037] (1) Configure zinc-iron alloy plating solution: prepare 1000mL plating solution, the composition of the plating solution is: ZnO 6g, NaOH 150g, triethanolamine 50mL, FeSO 4 ·7H 2 O 13g, dimethylamine and epichlorohydrin compound (DE) 6mL, ethylenediaminetetraacetic acid 0.6g, vanillin 0.1g, sodium dodecylsulfonate 0.1g, Zn powder 2g. Add 500mL water into the plating tank, pour solid NaOH into the water, stir and dissolve, then add NaOH solution to the ZnO that has been made into a paste while stirring. After it is completely dissolved, add water to 800mL, add 2g of Zn powder, stir for 30 minutes, and then clarify and filter. In a beaker, after diluting triethanolamine with 200 mL of water, FeSO 4 ·7H 2 O was added under stirring, and after it was completely dissolved, it was added to the plating tank, and then the compound (DE) o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com