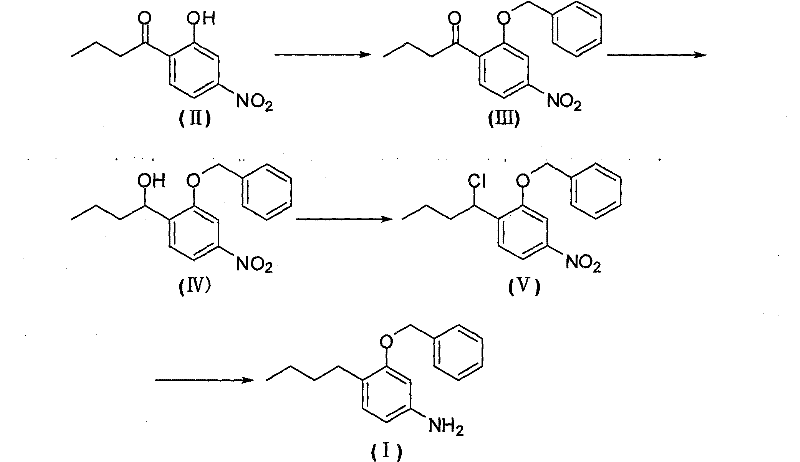
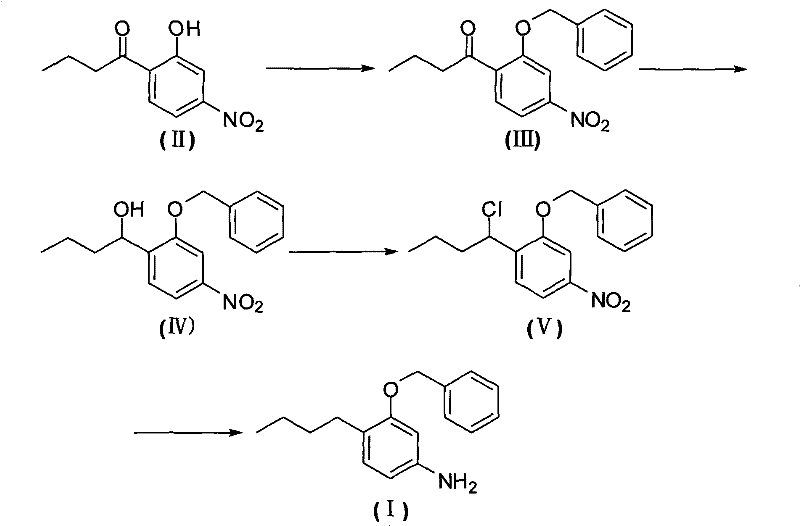
Method for preparing 3-butyl-4-benzyloxy-aniline
A technology of benzyloxyaniline and butyryl nitrobenzene is applied in the field of preparation of intermediates and can solve problems such as high cost, unsatisfactory yield and product purity, harsh conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A. Preparation of 3-benzyloxy-4-butyrylnitrobenzene
[0022] Add 2-butyryl-5-nitrophenol (20.9 g, 0.1 mol), DMF (N, N-di Methylformamide, 200g) and sodium carbonate (12.7g, about 0.12mol), stirred evenly and heated to 60°C, then slowly added benzyl chloride (14.0g, about 0.11mol) dropwise, and kept the reaction mixture after the dropwise addition The temperature was 60-70°C and the stirring reaction was continued for 4 hours, then ice water was added to precipitate a solid, which was collected by filtration and dried to obtain 3-benzyloxy-4-butyrylnitrobenzene (27.6g) with a yield of 92.3%.
[0023] B. Preparation of 1-(2-benzyloxy-4-nitrophenyl)-1-butanol
[0024] In a 500ml three-necked flask equipped with a reflux condenser, a thermometer and a magnetic stirring device, add 3-benzyloxy-4-butyrylnitrobenzene (29.9, 0.1mol), and the weight ratio concentration is 95% ethanol (300g ), after stirring evenly, add sodium borohydride (3.8g, 0.1mol) in batches, control the ...
Embodiment 2
[0031] Other steps are identical with embodiment 1, just the preparation method of the 3-benzyloxy-4-butyrylnitrobenzene of A step is as follows:
[0032] Add 2-butyryl-5-nitrophenol (20.9 g, 0.1 mol), DMF (N, N-di Methylformamide, 42g) and sodium carbonate (11.8g, about 0.11mol), stirred evenly and heated to 65°C, then slowly added benzyl chloride (12.8g, about 0.1mol) dropwise, and kept the reaction mixture after the dropwise addition The temperature was 60-70°C and the stirring reaction was continued for 4 hours, then ice water was added to precipitate a solid, which was collected by filtration and dried to obtain 3-benzyloxy-4-butyrylnitrobenzene (25.9g) with a yield of 86.6%.
Embodiment 3
[0034] Other steps are identical with embodiment 1, just the preparation method of the 3-benzyloxy-4-butyrylnitrobenzene of A step is as follows:
[0035]Add 2-butyryl-5-nitrophenol (20.9 g, 0.1 mol), DMF (N, N-di Methylformamide, 150g) and potassium carbonate (17.9g, about 0.13mol), after stirring evenly, the temperature was raised to 60°C, and then benzyl chloride (14.0g, about 0.11mol) was slowly added dropwise, and the reaction mixture was kept The temperature was 60-70°C and the stirring reaction was continued for 4 hours, then ice water was added to precipitate a solid, which was collected by filtration and dried to obtain 3-benzyloxy-4-butyrylnitrobenzene (27.4g) with a yield of 91.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com