Benzene-naphthalene diimide derivative, preparation method and its application
A naphthalene diimide and naphthalene imide technology, which is applied in the field of optoelectronic materials, can solve the problems of n-type carrier injection and transport materials with single types and narrow band gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
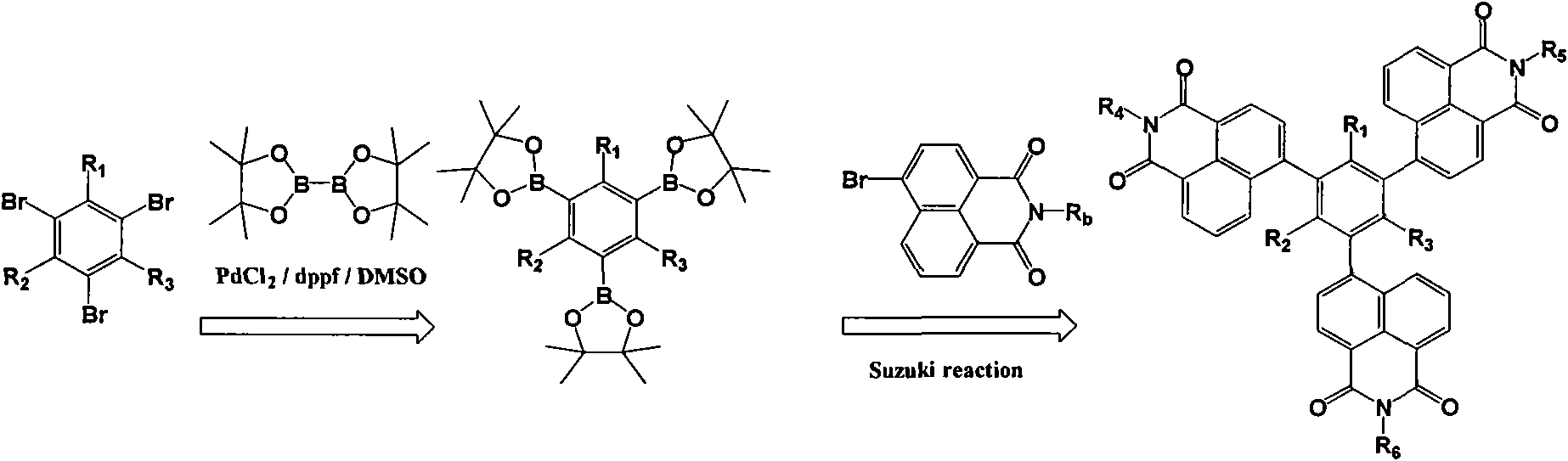
[0045] Embodiment 1: 1,3,5-three [(N'-butyl)-1 ', the synthesis of 8'-naphthalimide-4'-base] benzene
[0046] use as figure 1 Shown synthetic route prepares, specifically comprises the following steps:
[0047] (1), 1,3, the synthesis of 5-benzenetriboronic acid-tributanol ester
[0048] After passing nitrogen gas in a 250ml three-necked flask for 30min, add bisglutaryl boron (33mmol), appropriate amount of PdCl 2 With dppf, 90mmol potassium acetate, 1,3,5-bromobenzene (10mmol), and 120ml dimethyl sulfoxide (DMSO), heat and stir at 70°C until the raw material 1,3,5-bromobenzene disappears, and the reaction is terminated. After the reaction liquid was cooled to room temperature, it was filtered, and dimethyl sulfoxide (DMSO) was distilled from the filtrate under reduced pressure. The evaporated matter was washed with water with the filter cake, extracted with benzene, and the extract was dried with anhydrous magnesium sulfate. The solvent was distilled off by rotary distill...
Embodiment 2、1
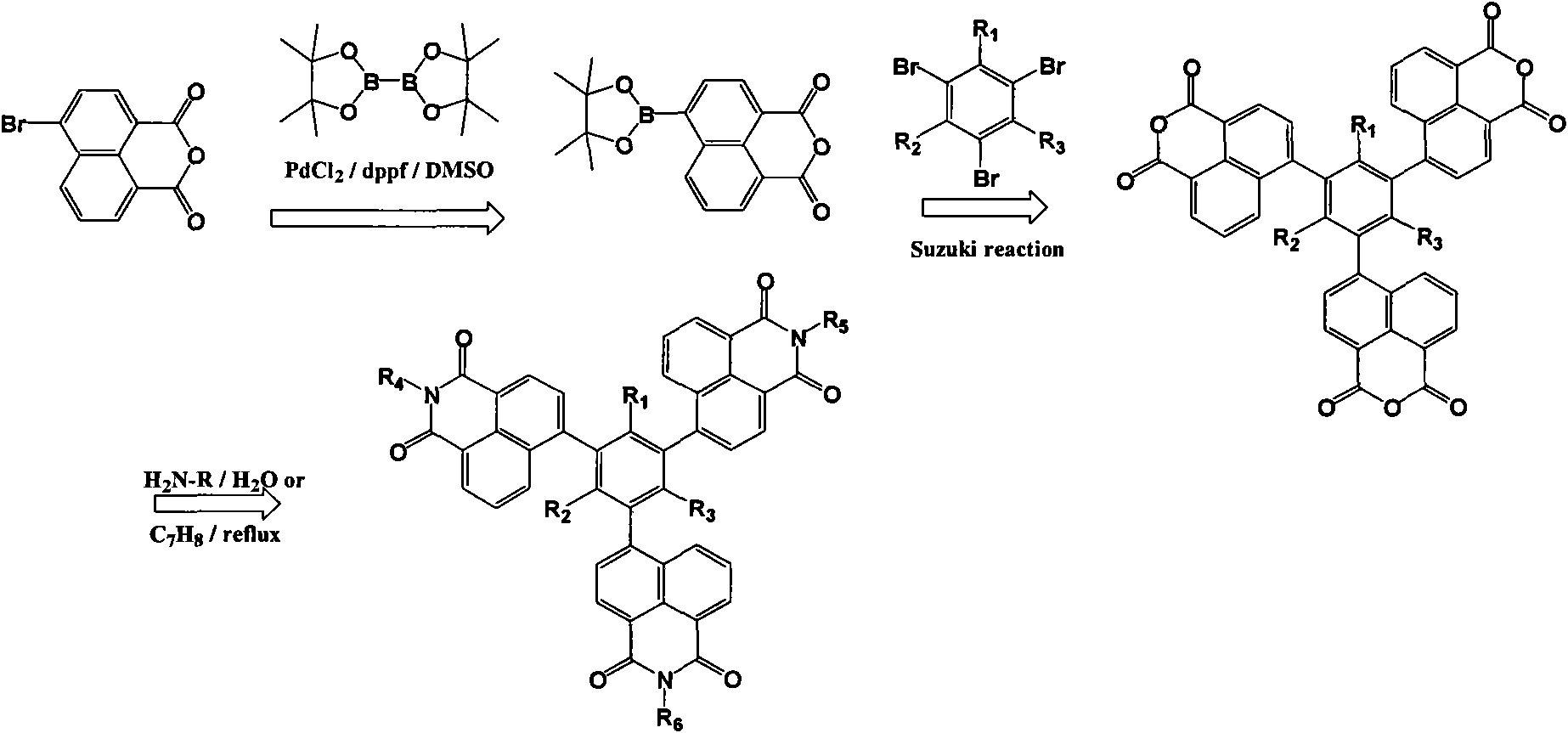
[0053] Example 2, 1,3,5-tri{[N'-(2"-ethylhexyl)]-1',8'-naphthylamide-4'-yl}benzene
[0054] use as figure 1 Shown synthetic route prepares, specifically comprises the following steps:
[0055] (1), 1,3, the synthesis of 5-benzenetriboronic acid-tributanol ester:
[0056] After passing nitrogen gas in a 250ml three-necked flask for 30min, add bisglutaryl boron (33mmol), appropriate amount of PdCl 2With dppf, 90mmol potassium acetate, 1,3,5-bromobenzene (10mmol), and 120ml dimethyl sulfoxide (DMSO), heat and stir at 150°C until the raw material 1,3,5-bromobenzene disappears, and the reaction is terminated. After the reaction liquid was cooled to room temperature, it was filtered, and dimethyl sulfoxide (DMSO) was distilled from the filtrate under reduced pressure. The evaporated matter was washed with water with the filter cake, extracted with benzene, and the extract was dried with anhydrous magnesium sulfate. The solvent was distilled off by rotary distillation, and the si...
Embodiment 3、1
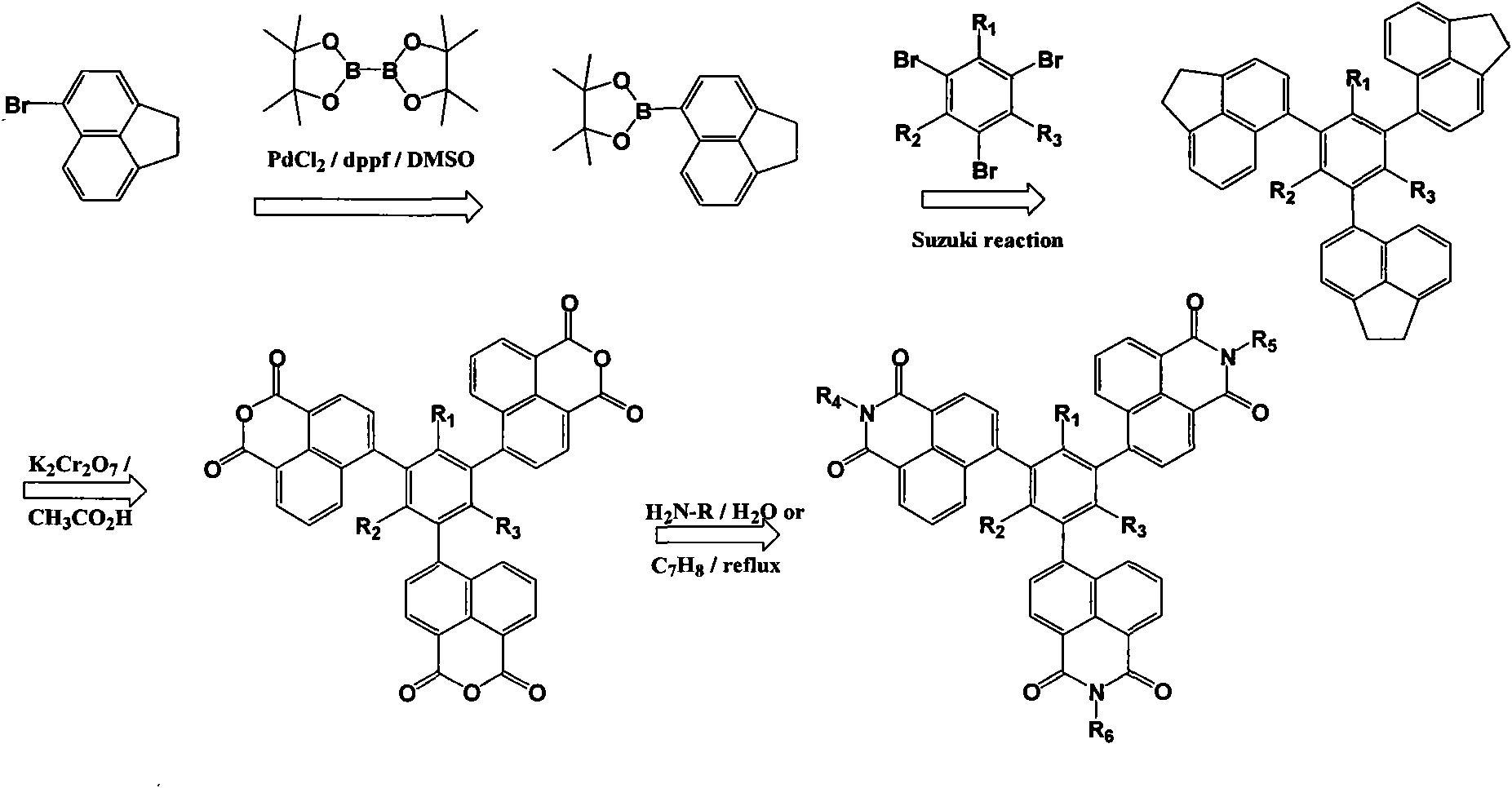
[0061] Example 3, 1,3,5-tri{[N'-(9", 9"-dibutylfluorene-2"-yl)]-1', 8'-naphthylamide-4'-yl}benzene
[0062] use as figure 1 The synthetic route shown specifically comprises the following steps:
[0063] (1), 1,3, the synthesis of 5-benzenetriboronic acid-tributanol ester:
[0064] After passing nitrogen gas in a 250ml three-necked flask for 30min, add bisglutaryl boron (33mmol), appropriate amount of PdCl 2 With dppf, 90mmol potassium acetate, 1,3,5-bromobenzene (10mmol), and 120ml dimethyl sulfoxide (DMSO), stir at 20°C until the raw material 1,3,5-bromobenzene disappears, and the reaction is terminated. After filtration, the filtrate was distilled off under reduced pressure to remove dimethyl sulfoxide (DMSO). The evaporated matter was washed with water with the filter cake, extracted with benzene, and the extract was dried with anhydrous magnesium sulfate. The solvent was distilled off by rotary distillation, and silica gel column chromatography was eluted with a mixed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com