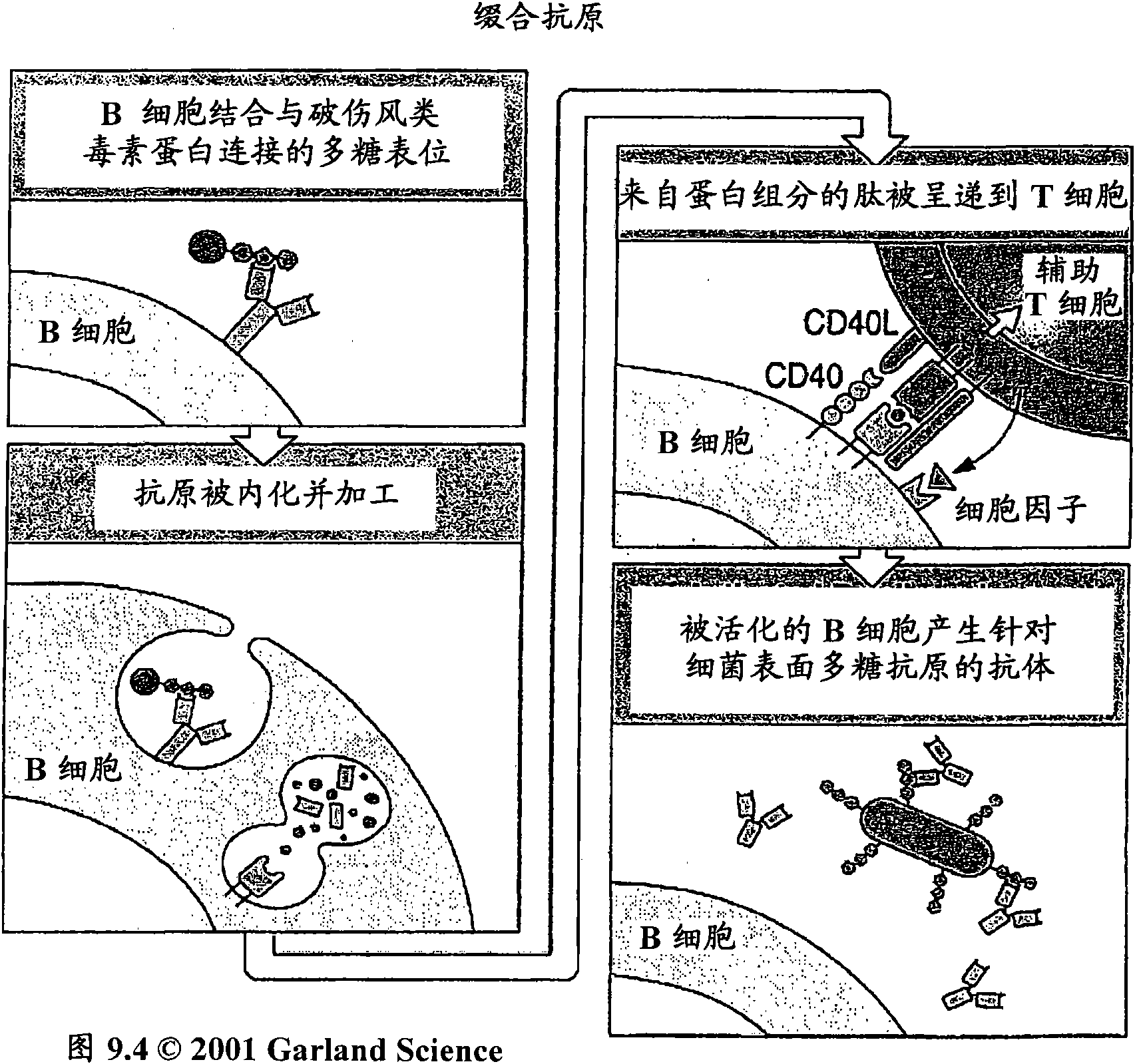
Protein matrix vaccines and methods of making and administering such vaccines
A protein and carrier protein technology, applied in the field of vaccine compositions, can solve the problems of little immune response stimulation, no stimulation, complicated efforts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0188] Example 1. Vaccine and control formulations
[0189] Capsular poly-γ-D-glutamic acid (PGA) was purchased from Vedan (Taiwan) or purified by the method of Rhie et al. (Proc. Natl. Acad. Sci. USA 100: 10925-10930, 2003). Dominant negative mutant (DNI) is a mutant form of Bacillus anthracis (B. anthracis) protective antigen (PA), prepared from Escherichia coli (Escherichia coli) by the method of Benson et al. (Biochemistry 37:3941-3948, 1998) have to. PGA and DNI proteins were extensively dialyzed against 0.05M, pH 7.4 sodium phosphate buffer (SP7.4) before use. The concentration of DNI stock solution is 30mg / mL. The PGA stock solution concentration was 134 mg / mL. Linker glutaraldehyde was purchased from Pierce as a 25% stock solution. Protein capsular matrix vaccine (PCMV) and controls were assembled in the reactions in Table 1.
[0190] Table 1. Assembly of reactions used to prepare PCMV formulations 1-3 and controls 4 and 5
[0191] Reaction #DNI PGA dH 2 O 25% g...
Embodiment 2
[0210] Example 2. Immunization and analysis of anti-DNI and anti-PGA immune responses
[0211] The soluble products of the five reactions described in Table 1 were adjusted to the same protein concentration (based on their absorbance at 280 nm). BALB / c mice approximately 5-7 weeks old from Charles River were used for figure 2 All immunization studies described. Mice were immunized with PCMV vaccines 1-3 and antigen preparation controls 4 and 5 at a dose of 20 μg of DNI protein by intraperitoneal injection on day 0. All mice were bled on day 7 and then boosted with the same dose of antigen preparation on day 10. Mice were bled again on day 17 and boosted again on day 20. Mice were bled again on day 30 and sacrificed this time. Serum was collected from blood samples after clotting and stored at -20°C. The levels of anti-PGA serum antibodies and anti-DNI serum antibodies were analyzed using enzyme-linked immunosorbent assay (ELISA). Briefly, Immulon 2HB ELISA (VWR) microti...
Embodiment 3
[0215] Example 3. Preparation and Characterization of Additional PCMVs
[0216] PCMV technology can be applied to capsular antigens of various structures and ionic charges. 23 types of Streptococcus pneumonia PS were purchased from American Type Culture Collection (ATCC) and manufactured by Merck, Inc. These PSs vary widely in their molecular structures, including strongly anionic, partially cationic, neutrally charged, phosphorylated, linear, branched, and modified in various other ways. In a pilot experiment, a subset of these PSs (4, 6B, 9V, 14, 18C, 19F, and 23F) corresponding to the seven capsular types in the Wyeth product Prevnar were taken and determined to induce IL-6 production by mouse macrophages Ability. Type 4 PS was active in the assay; lipopolysaccharide (LPS) was the control for TLR agonists. Other PS (eg, type 3), PGA and O antigen PS from F. tularensis, as well as PCMV vaccines prepared from PGA-DNI and no cross-linked controls were also assayed. This ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com