Functional graphene-polymer nanocomposites for gas barrier applications
A graphene, functional technology, applied in the field of barrier films and gas diffusion films, which can solve the problem of ineffective production of polymer nanocomposites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0147] The mechanical properties of the NR-FG nanocomposites were characterized by tensile measurements. Figure 8 shows the stress-strain curves for pure NR and filled NR composites with different loadings of FG and carbon black (CB). It can be seen that FG-filled NR shows higher mechanical properties compared to pure NR and 20 phr (parts in one hundred) CB. At 3phr FG loading, the Young's modulus and stress at 400% elongation were improved by more than 7 times compared with pure NR. At 20phr CB loading, the Young's modulus improvement was only twofold; this was achieved with only 1phr FG loading. This level of reinforcement is superior compared to clay and SWCNT filled elastomers. In one embodiment, the high surface area of FG and the presence of functional groups on the surface enhance the mechanical properties of the nanocomposite.
[0148] In preferred embodiments, SBR and PS-PI-PS also exhibit improved mechanical properties. For example, at 2phr FG loading, the modu...
Embodiment 1
[0180] Graphite oxide is prepared from graphite by an oxidation and intercalation process known as the Staudenmaier method. The method employs a combination of oxidizers and intercalants: sulfuric acid, nitric acid, and potassium chlorate under controlled temperature conditions. A ratio of graphite to potassium chlorate in the range of 1:8 to 1:20 (wt / wt) is preferred. A ratio of sulfuric acid to nitric acid of from 5:1 to 1:1 is preferred. The Staudenmaier method is the preferred oxidation method.
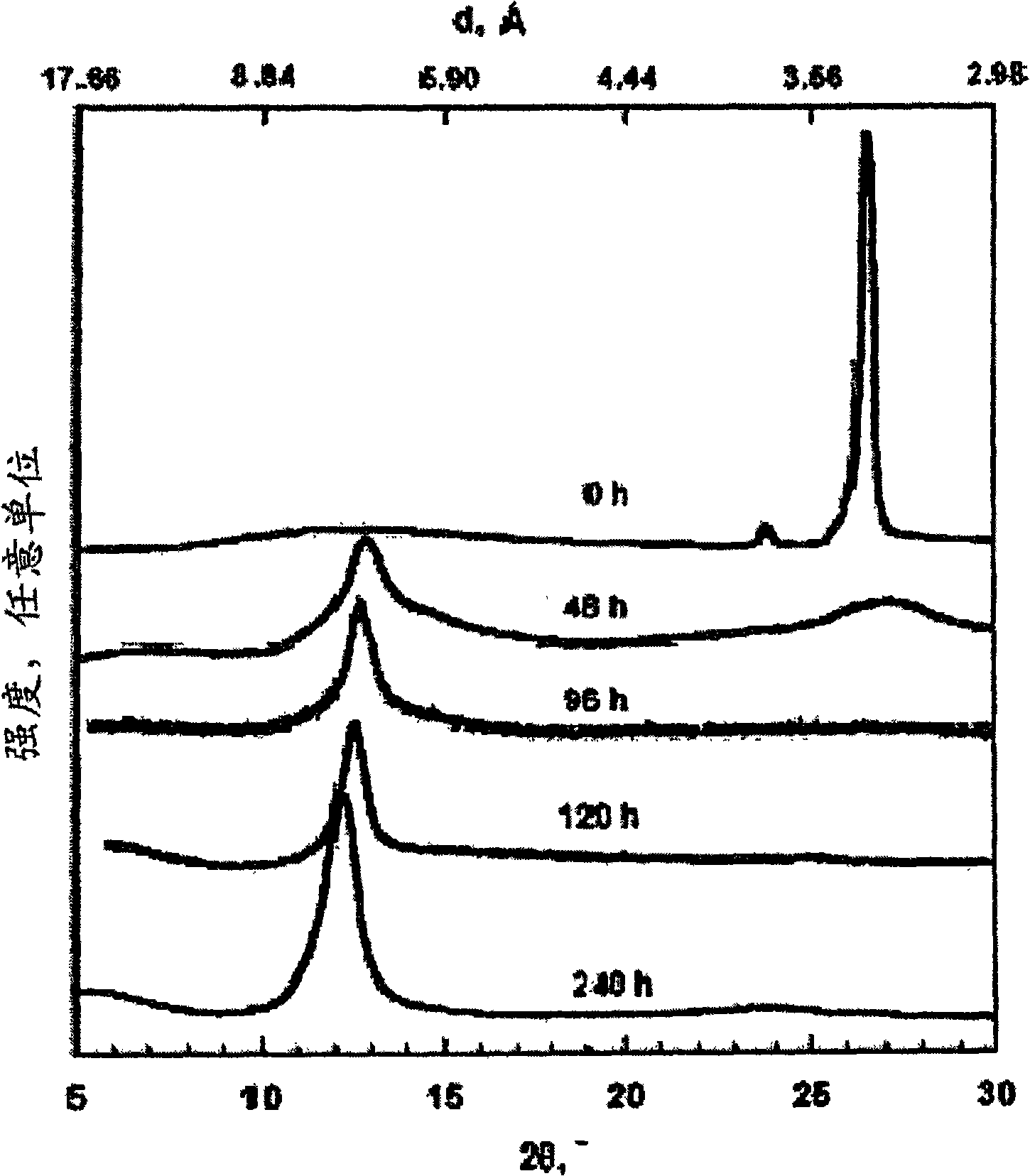
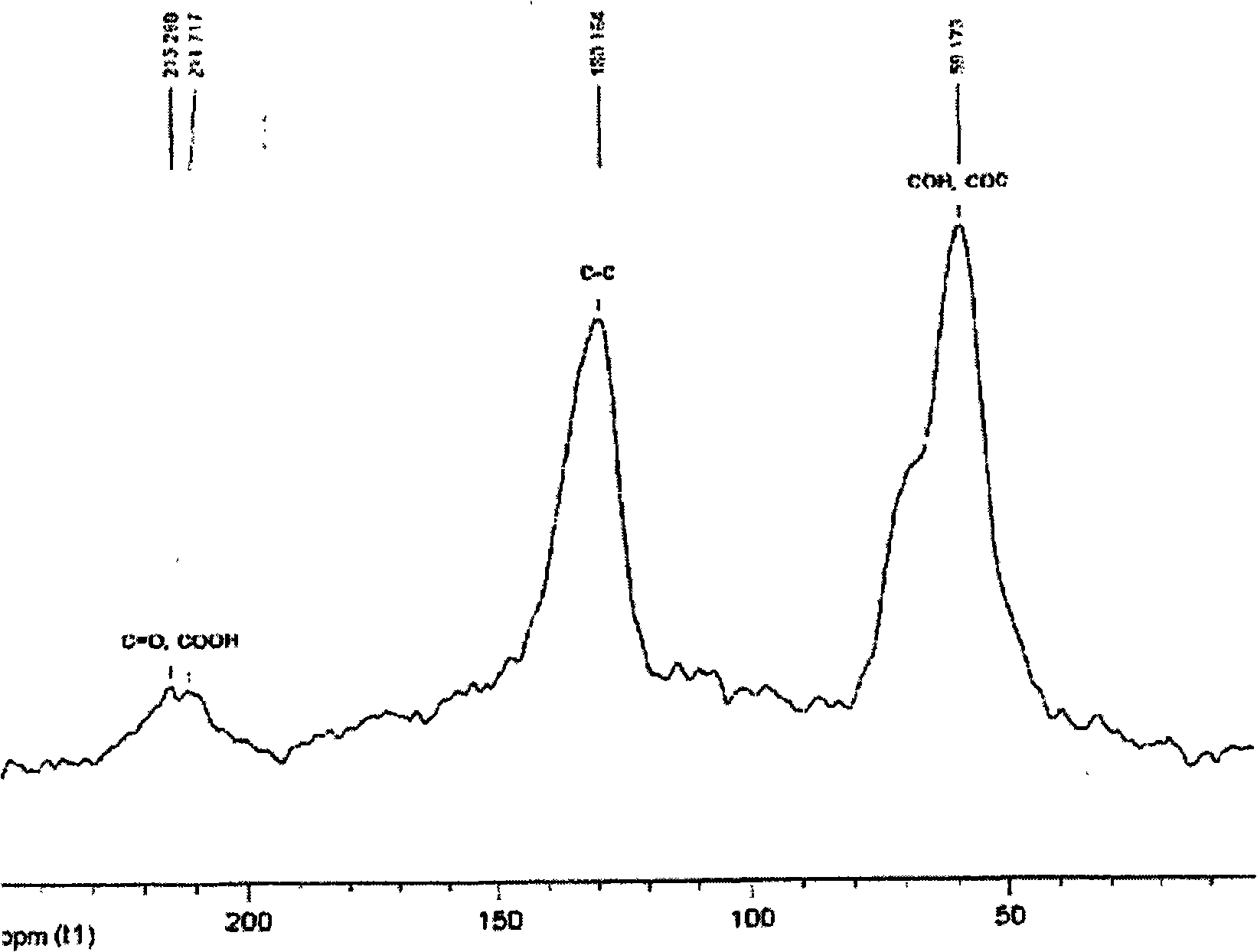
[0181] In this example, 5 g of graphite flakes (Asbury Carbon, Asbury, NJ) with an average sheet diameter of 400 μm (Asbury Carbon, Asbury, NJ) were added to an ice-cooled solution containing 85 ml of sulfuric acid and 45 ml of nitric acid. This was followed by the addition of 55 g of potassium chlorate over 20 minutes so that the temperature did not exceed 20°C. After the oxidation / intercalation process was performed for 96 hours, the reaction mixture was poured into 7 1 of de...
Embodiment 2
[0183] In the preparation of thermally exfoliated graphite oxide (FGS), graphite oxide (0.2 g) was placed in a porcelain boat and inserted into a quartz tube with an inner diameter of 25 mm and a length of 1.3 m closed at one end. The other end of the quartz tube was closed with a rubber stopper. The argon (Ar) inlet and thermocouple were then inserted through the rubber plug. The sample was purged with Ar for 10 minutes; the quartz tube was then quickly inserted into a preheated Lindberg tube furnace and heated for 30 seconds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent density | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| tensile modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com