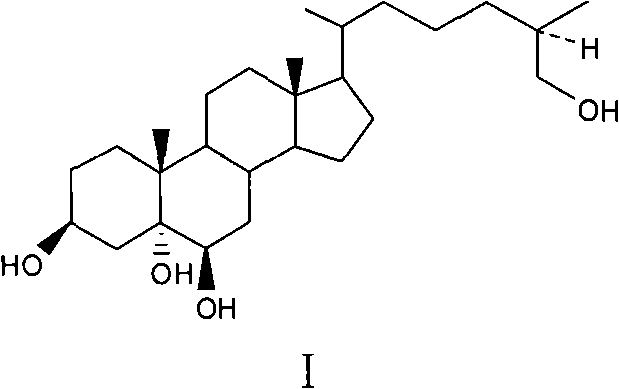
Method for synthesizing marine polyhydroxyl sterol (25R)-5alpha -cholest-3 beta, 5alpha, 6beta, 26-tetrol
A technology of marine sterols and synthetic methods, which is applied in the field of synthesis of polyhydroxy marine sterols, can solve the problem of high extraction costs, and achieve the effects of scientific and reasonable synthetic routes, good selectivity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
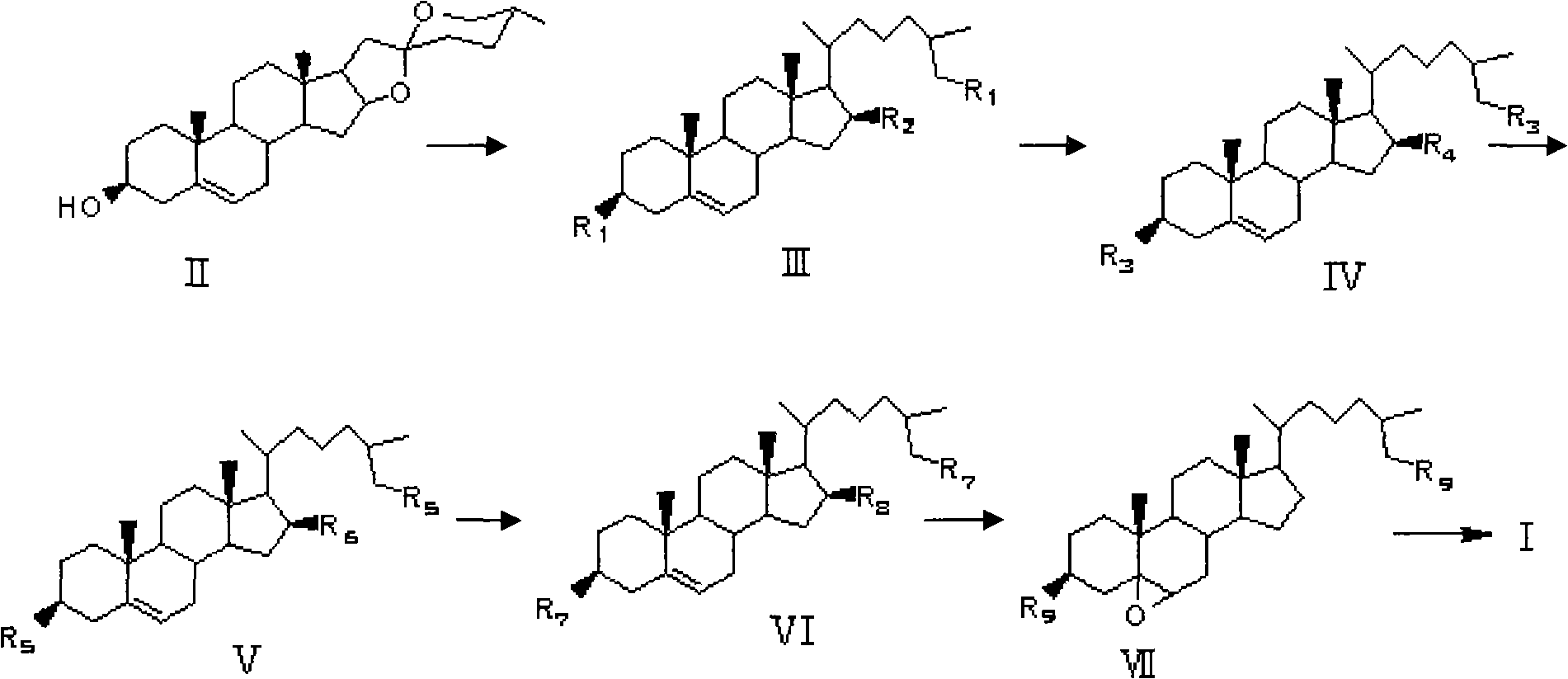
[0031] The synthesis method of the polyhydroxy marine sterol (25R)-5α-cholesta-3β, 5α, 6β, 26-tetrol provided in this example is as follows:
[0032] (1) Add compound II (15g, 37mmol) and absolute ethanol 1000ml in a 2L reaction flask, heat and reflux at 95°C to completely dissolve the saponin in ethanol, add 585g of zinc amalgam while it is hot, and use a constant pressure funnel to Concentrated HCl (400ml, 37.5%, w / w) was added to the reaction system, dripped in about 1h, continued to stir and heat to reflux for 4h, the reaction solution was concentrated under reduced pressure to remove ethanol, extracted 3 times with 150ml ethyl acetate respectively, organic The layer was washed with water until neutral, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain 10.8 g of white powder Compound III with a yield of 72%;
[0033] (2) Dissolve compound III (30g, 71.66mmol) in 300ml of anhydrous DMF, add i...
Embodiment 2
[0039] The synthesis method of the polyhydroxy marine sterol (25R)-5α-cholesta-3β, 5α, 6β, 26-tetrol provided in this example is as follows:
[0040] (1) Add compound II (15g, 37mmol) and absolute ethanol 1000ml in a 2L reaction flask, heat and reflux at 95°C to completely dissolve the saponin in ethanol, add 445g of zinc amalgam while it is hot, and use a constant pressure funnel to Add concentrated HCl (300ml, 37.5%, w / w) to the reaction system, follow the reaction by TLC, stop the reaction after the disappearance of the raw materials, filter, remove most of the ethanol under reduced pressure, extract 1 to 3 times with dichloromethane, and wash the organic layer with water to neutrality, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 13 g of white solid III;
[0041] Steps (2) to (6) in this embodiment are the same as steps (2) to (6) in Embodiment 1.
Embodiment 3
[0043] The synthesis method of the polyhydroxy marine sterol (25R)-5α-cholesta-3β, 5α, 6β, 26-tetrol provided in this example is as follows:
[0044] Steps (1) to (3) of this embodiment are identical to steps (1) to (3) in Example 1;
[0045] (4) Dissolve compound V (22.5g, 31.00mmol) in 440ml of anhydrous tetrahydrofuran, slowly add lithium aluminum hydride (3.51g, 91mmol) in batches and stir at room temperature for 16h. Sodium sulfate quenched the reaction, the reaction solution was white and turbid, washed with 5-10% (w / w) dilute hydrochloric acid, and the organic layer was washed with water until neutral, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 16.0 g of white powder VI, yield 83%;
[0046] (5) Dissolve compound VI (8g, 12.67mmol) in 480ml of dichloromethane, add 240ml of water and sodium carbonate (6g, 72.29mmol), slowly add m-chloroperbenzoic acid (8.67g, 50.24mmol) under stirring at room temperature ), finish the reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com