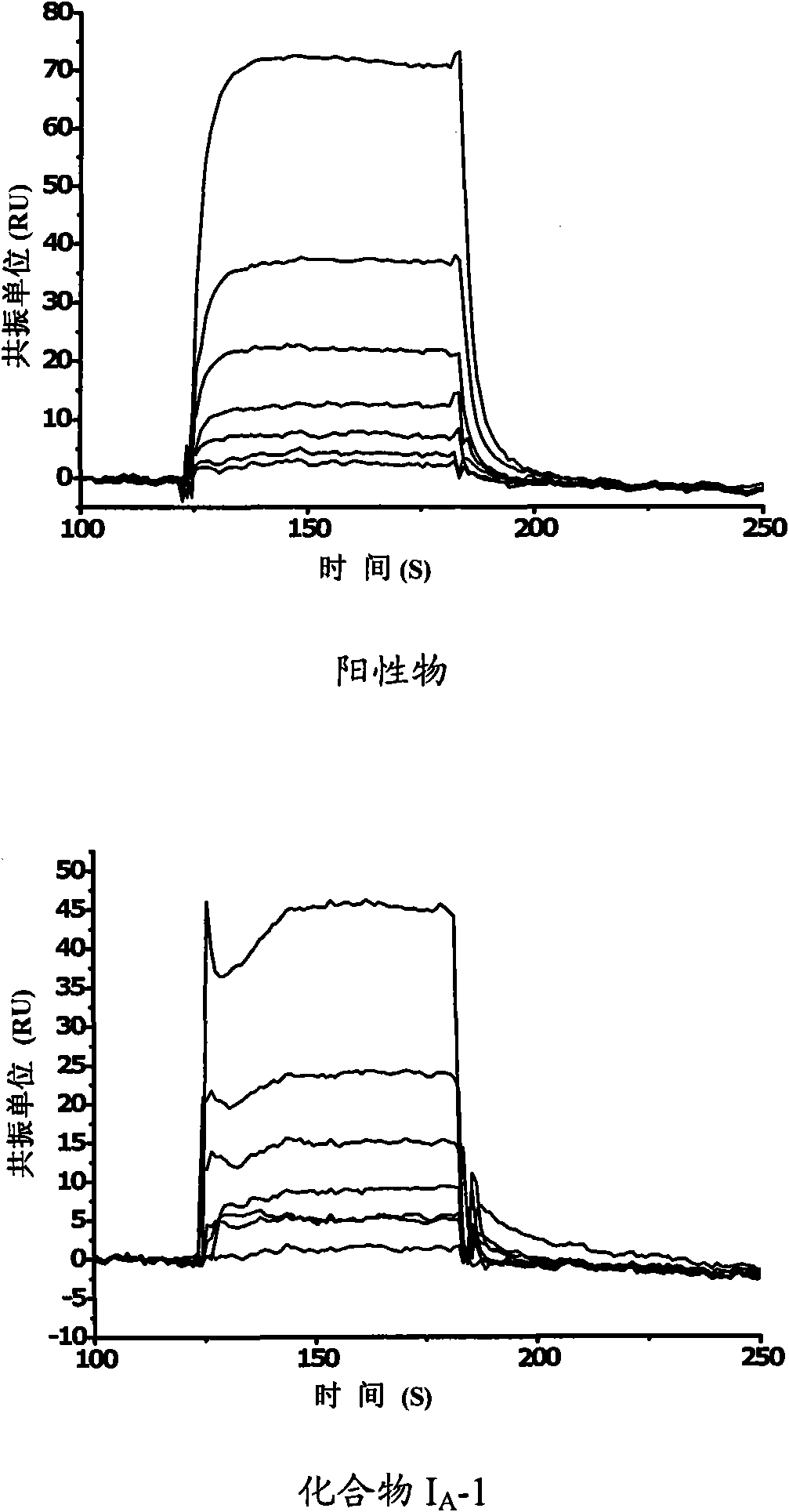
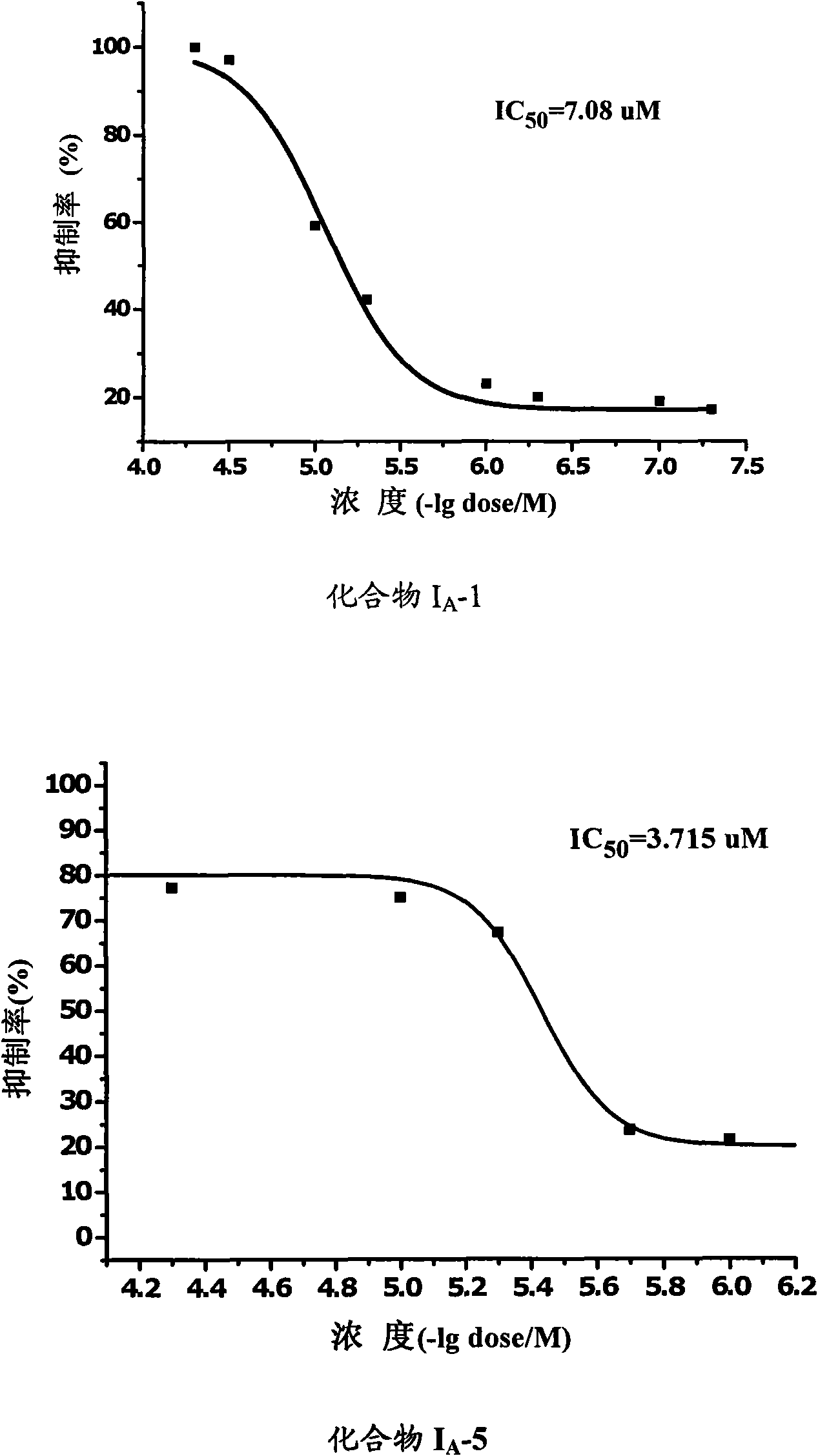
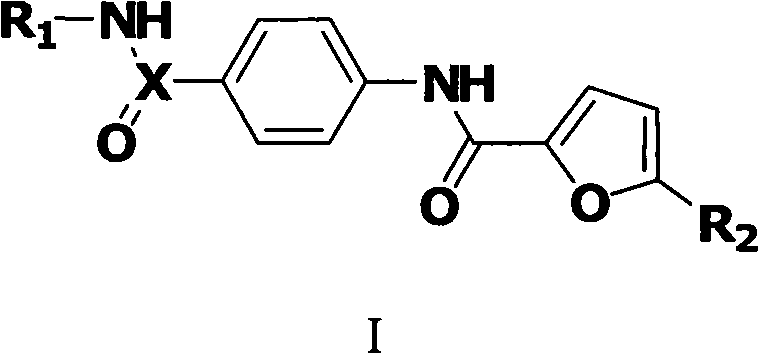
Amide derivative and purpose thereof
A technology of amide derivatives and formamide, applied in the field of amide derivatives, can solve problems such as affecting the function of oocysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Formula I A Preparation of the indicated compounds:
[0037] 1) Methyl 5-chloromethylfuran-2-carboxylate, R 3 OH (such as substituted aryl phenol), inorganic base and organic solvent are mixed, reflux overnight; most of the solvent is evaporated, water is added, organic solvent is extracted, dried, and the solvent is evaporated to dryness to obtain 5-(substituted aryloxymethyl)furan- Methyl 2-formate (compound represented by formula II).
[0038] 2) Put the compound II and inorganic base obtained in step 1) into an appropriate amount of methanol, and stir overnight at 50°C; evaporate most of the solvent, add water, adjust the pH to 1 with hydrochloric acid, precipitate a solid, and filter with suction to obtain 5-(substituted aryloxymethyl)furan-2-carboxylic acid (compound represented by formula III).
[0039] 3) R at room temperature 1 The substituted amine is reacted with p-acetamidobenzenesulfonyl chloride in an alkaline solution at a temperature of 40°C to 45°C ...
Embodiment 1
[0051] Preparation of methyl 5-(4-chloro-3-methylphenoxymethyl)furan-2-carboxylate (compound II-1)
[0052] Mix 2.5 g of 5-chloromethylfuran-2-methylcarboxylate, 2.2 g of 4-chloro-3-methylphenol, 2.6 g of potassium carbonate and 40 ml of acetonitrile, and reflux overnight; evaporate most of the solvent, add water, acetic acid Ethyl ester was extracted, the ester layer was dried, and the solvent was evaporated to dryness to obtain 3.8 g of white solid with a yield of 94.5%. mp 56-58°C; 1 H-NMR (DMSO-d 6 , 500MHz) δ2.29(s, 3H), 3.81(s, 3H), 5.14(s, 2H), 6.80(d, 1H), 6.91(dd, 1H), 7.07(d, 1H), 7.20(d , 1H), 7.30-7.33 (m, 2H); MS (EI) m / z 280 (M + ).
Embodiment 2
[0054] Preparation of 5-(4-chloro-3-methylphenoxymethyl)furan-2-carboxylic acid (compound III-1)
[0055] Put 1.7 g of II-1 and 0.5 g of lithium hydroxide monohydrate into 20 ml of methanol, and stir overnight at 50°C. Most of the solvent was evaporated, water was added, the pH was adjusted to 1 with hydrochloric acid, a solid was precipitated, and 1.58 g of white solid (III-1) was obtained by suction filtration, with a yield of 97.5%. mp 194-195°C; 1 H-NMR (DMSO-d 6 , 500MHz) δ2.28(s, 3H), 5.11(s, 2H), 6.74(d, 1H), 6.89(dd, 1H), 7.05(d, 1H), 7.20(d, 1H), 7.30(d , 1H); MS (EI) m / z 266 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com