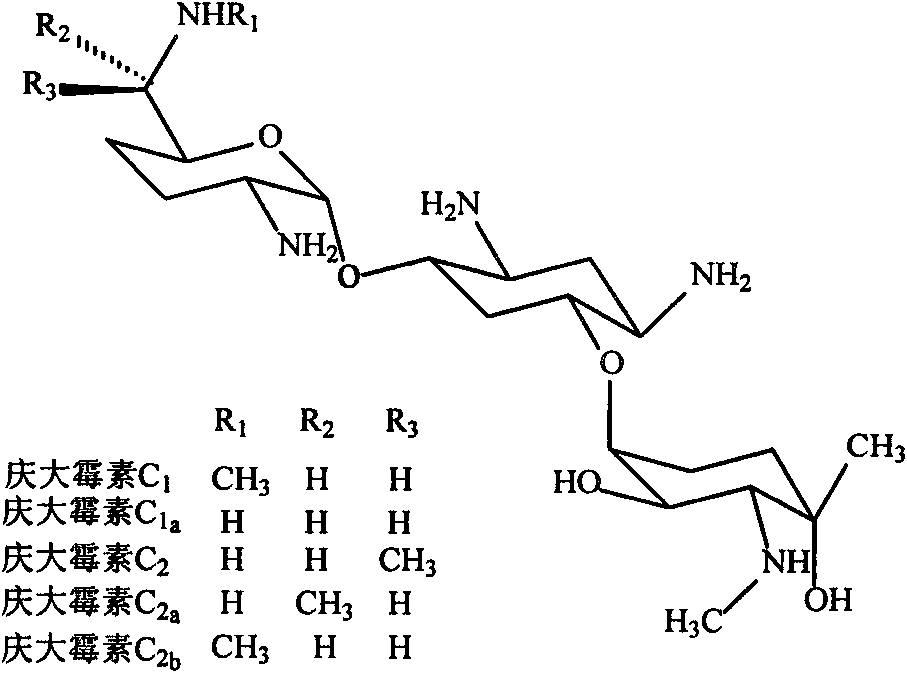
Method for detecting gentamicin through clinical magnetic resonance imaging
A technology of magnetic resonance imaging and gentamicin, applied in the field of immunoassay, achieves the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 The coupling of gentamicin and magnetic nanoparticles:
[0028] Add 1 μL carbodiimide EDC and 2.4 mg N-hydroxysulfosuccinimide sulfo-NHS to 0.5 mL, 1.3 mg / mL Fe 3 o 4 In aqueous solution, the reaction takes about 15 minutes to activate. Activated Fe 3 o 4 Add 1mg of gentamycin (GM drug), react at room temperature for 4 hours, and dialyze in 0.01M, pH 7.4 PBS for 24 hours to remove unreacted small drug molecules.
Embodiment 2
[0029] Embodiment 2 magnetic resonance:
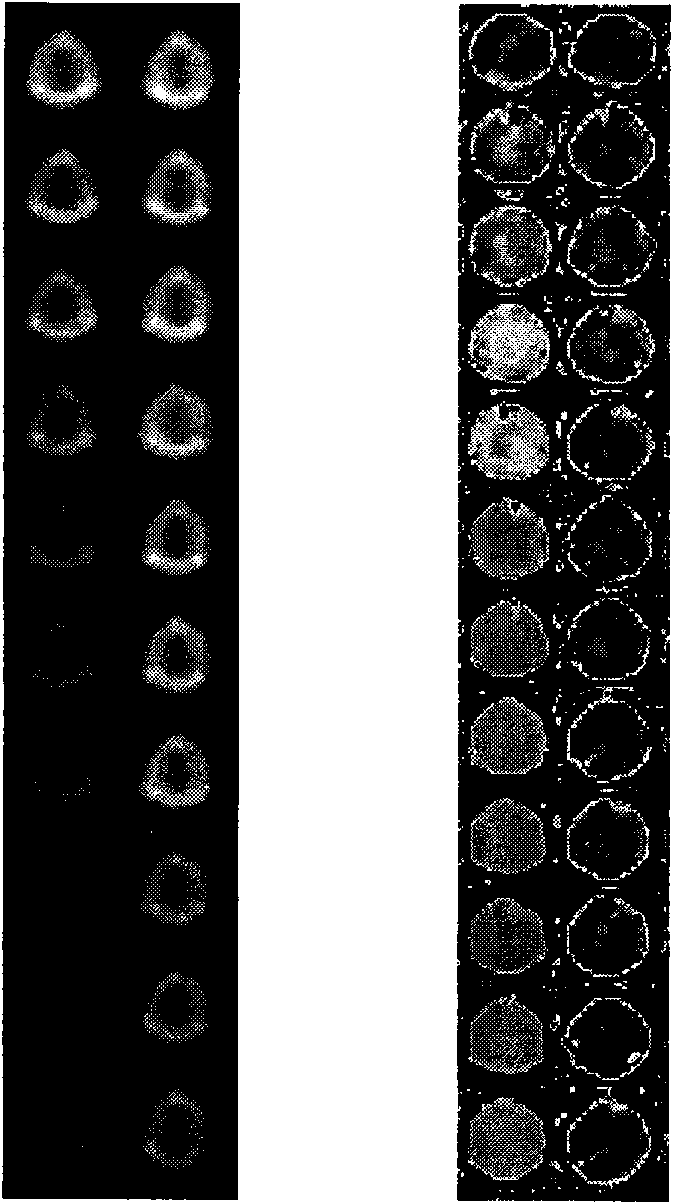
[0030] 20 μL, 150 μg / mL of gentamicin-modified Fe 3 o 4 Add 20 μL of different concentrations of gentamicin standard, add 1 μL of gentamicin antibody (5 mg / mL), then add 1 μL of goat anti-rabbit secondary antibody, incubate for 2 hours, dilute to a volume of 250 μL, and add to the microwell plate, measured by MRI.
[0031] Control group settings: gentamicin antibody was replaced by dexamethasone antibody, and other operations were the same as above.
[0032] The solutions used were all 0.01M PBS buffer, pH 7.4. The concentrations of gentamicin standard substances were 0, 8, 12, 16, 32, 48, 72, 100, 120, 144ng / mL, respectively.
Embodiment 3
[0033] Embodiment 3 Magnetic resonance quantitative detection
[0034] The magnetic resonance equipment is MAGNETOM Avanto 1.5T MRI, Siemens clinical magnetic resonance imaging equipment.
[0035] The scanning time parameters used in this experiment are: echo time (TE) is 20-120ms, pulse repetition time (repetition time) TR is 2000ms, and the coil used is the head coil.
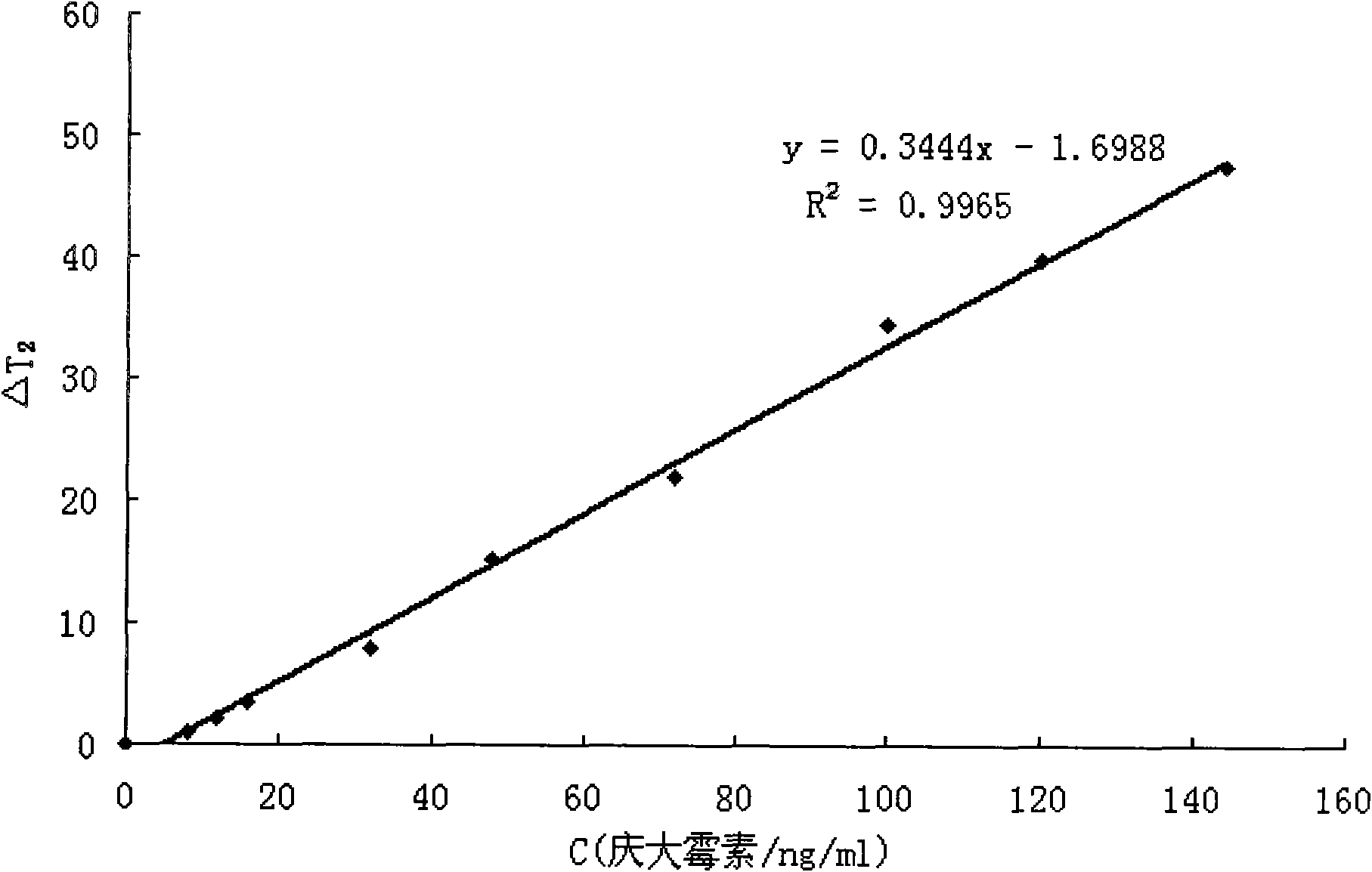
[0036] The principle of magnetic resonance detection of target is to induce water proton T through the formation of antibody and drug-modified magnetic nanoparticle immune polymer 2 The size of the value can achieve the purpose of detecting the target object. The more gentamicin added to the water, the less polymer is formed, T 2 The value is larger. established by T 2 ~C / gentamicin concentration standard curve can be compared to obtain the concentration of gentamicin in the sample. See attached for standard curve image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com