New use of glyoxal bis (thiosemicarbazone) compound
A technology of glyoxal-based bisthiosemicarbazone and dialdehyde-based bisthiosemicarbazide is applied in the directions of medical preparations, drug combinations, and pharmaceutical formulations containing active ingredients, and achieves low toxic and side effects, strong killing effect, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1, compound and preparation thereof
[0065] 1. 2-Oxobutyraldehyde bisthiosemicarbazone (IMMLG-597) and its preparation
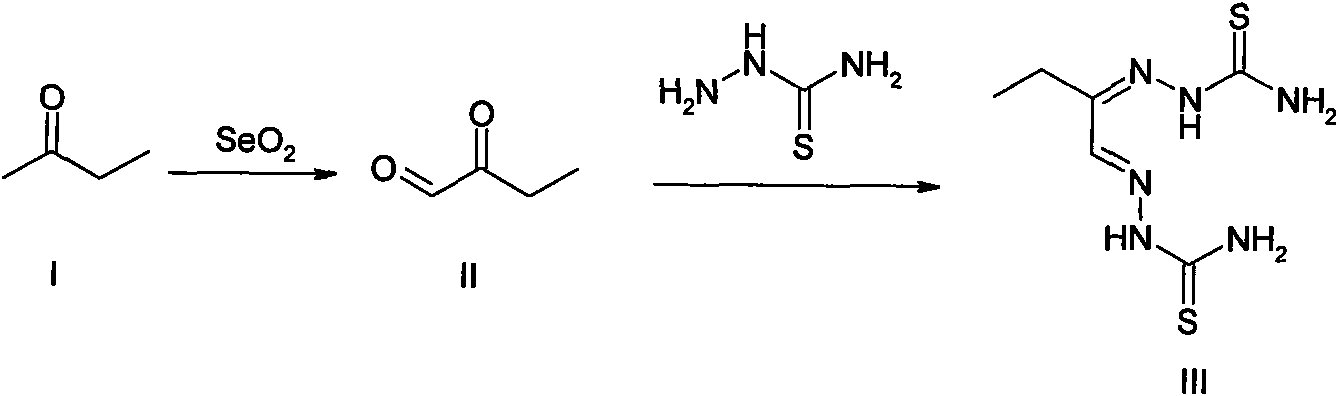
[0066] (1) Preparation method: the preparation process is as follows: figure 1 shown.
[0067] Step 1: Synthesis of 2-oxobutyraldehyde: In a 250 mL round bottom flask, add equimolar amounts of 2-butanone and selenium dioxide (0.25 mol), and then add 180 mL of dioxane and 12 mL of deionized water. Heating to reflux for 6 hours (until the precipitation of selenium close to the theoretical amount). Selenium was removed by filtration while it was hot, and after the solvent was evaporated, an oil pump was distilled under reduced pressure to obtain 6.4 g of golden yellow liquid 2-oxobutyraldehyde, with a yield of 30%. Step 2: Synthesis of 2-oxobutyraldehyde bisthiosemicarbazone (IMMLG-597): Add 2.2mol thiosemicarbazide and 10mL 5% glacial acetic acid-95% ethanol (v / v) solution in a 50mL round bottom flask , control the reaction temperature a...
Embodiment 2
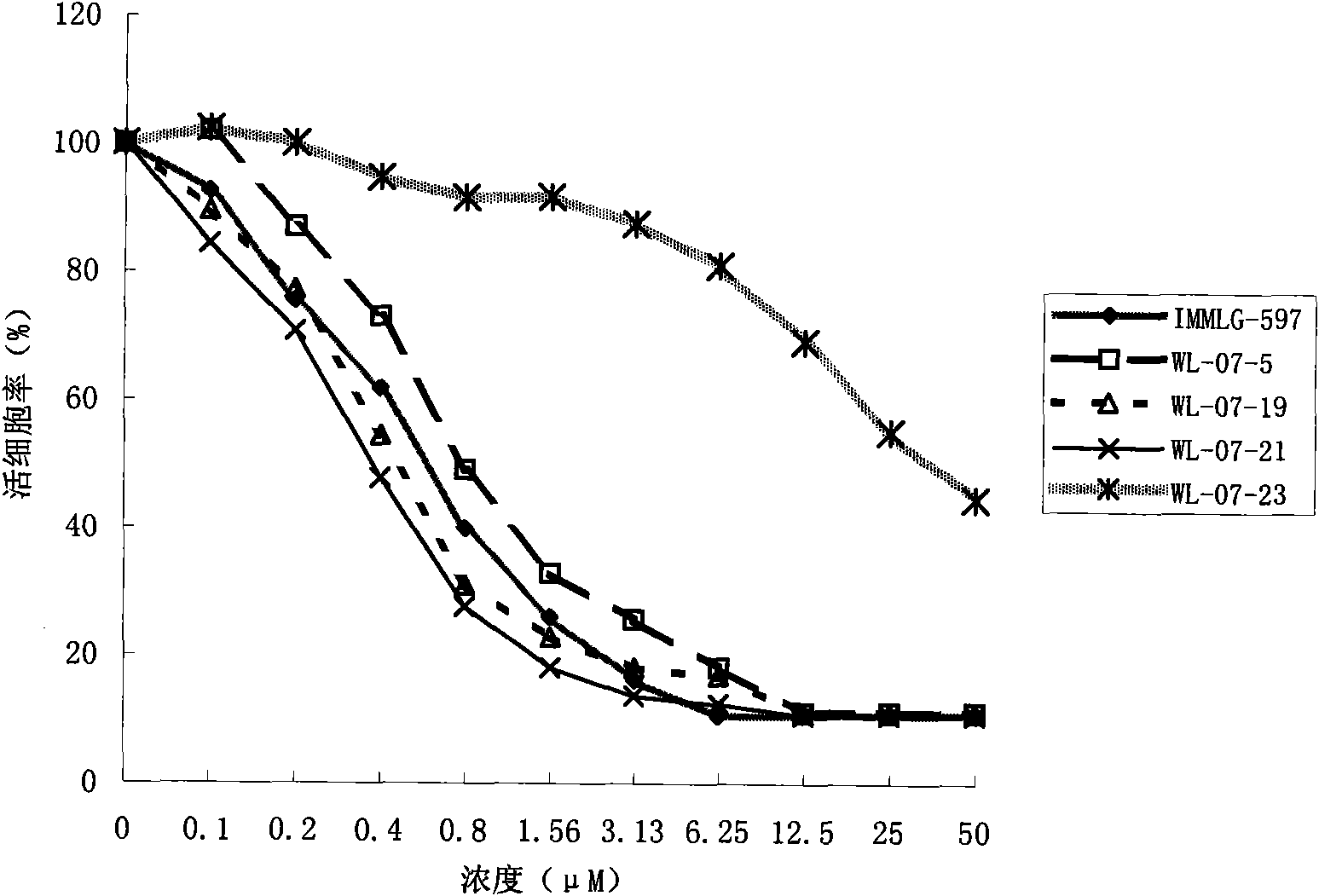
[0081] Embodiment 2, five kinds of compounds are to the inhibitory activity of tumor cell
[0082] BEL7402 liver cancer cells and HepG2 liver cancer cells overexpressing the LAPTM4B gene and its encoded LAPTM4B-35 protein were used to screen 1697 small compounds in the combinatorial chemical library for their killing effect on cancer cells. A normal human fetal liver cell line with low expression of the LAPTM4B gene and its encoded LAPTM4B-35 protein was used as a control. Acid phosphatase assay (APA) was used to measure the rate of viable cells to detect the inhibitory / killing activity of each compound on tumor cells.
[0083] Use DMEM medium containing 10% newborn bovine serum (10% fetal bovine serum for fetal liver cells) for the cells to be tested, at saturated humidity, 37°C, 5% CO 2 cultured in an incubator. Cells in the logarithmic growth phase cultured in vitro were digested with 0.25% crude trypsin to make a single cell suspension (adjust the cell concentration to 3...
Embodiment 3
[0089] Example 3, IMMLG-597 compound's in vitro killing effect on tumor cells and in vivo tumor inhibiting effect
[0090] 1. In vitro tumor cell killing activity of IMMLG-597
[0091] 1. Calcein AM / EthD-1 fluorescence double staining simultaneously detects dead cells (red fluorescence) and living cells (green fluorescence) and cells entering middle / late stage apoptosis (red fluorescence):
[0092] HepG2 liver cancer cell line and FLC fetal liver cell line were used as experimental cells. The above-mentioned cells in the logarithmic growth phase were digested with 0.25% crude trypsin, washed with PBS, and made into a single-cell suspension with DMEM medium containing 10% newborn bovine serum, and adjusted the cell density (according to different cell types, it can be between 3-6 ×10 4 cells / ml) in 96-well plate (3~6×10 3 cells / 100 μl / well); normal human fetal liver FLC cells were cultured in DMEM medium containing 10% fetal bovine serum. Cells to be tested in 5% CO 21. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com