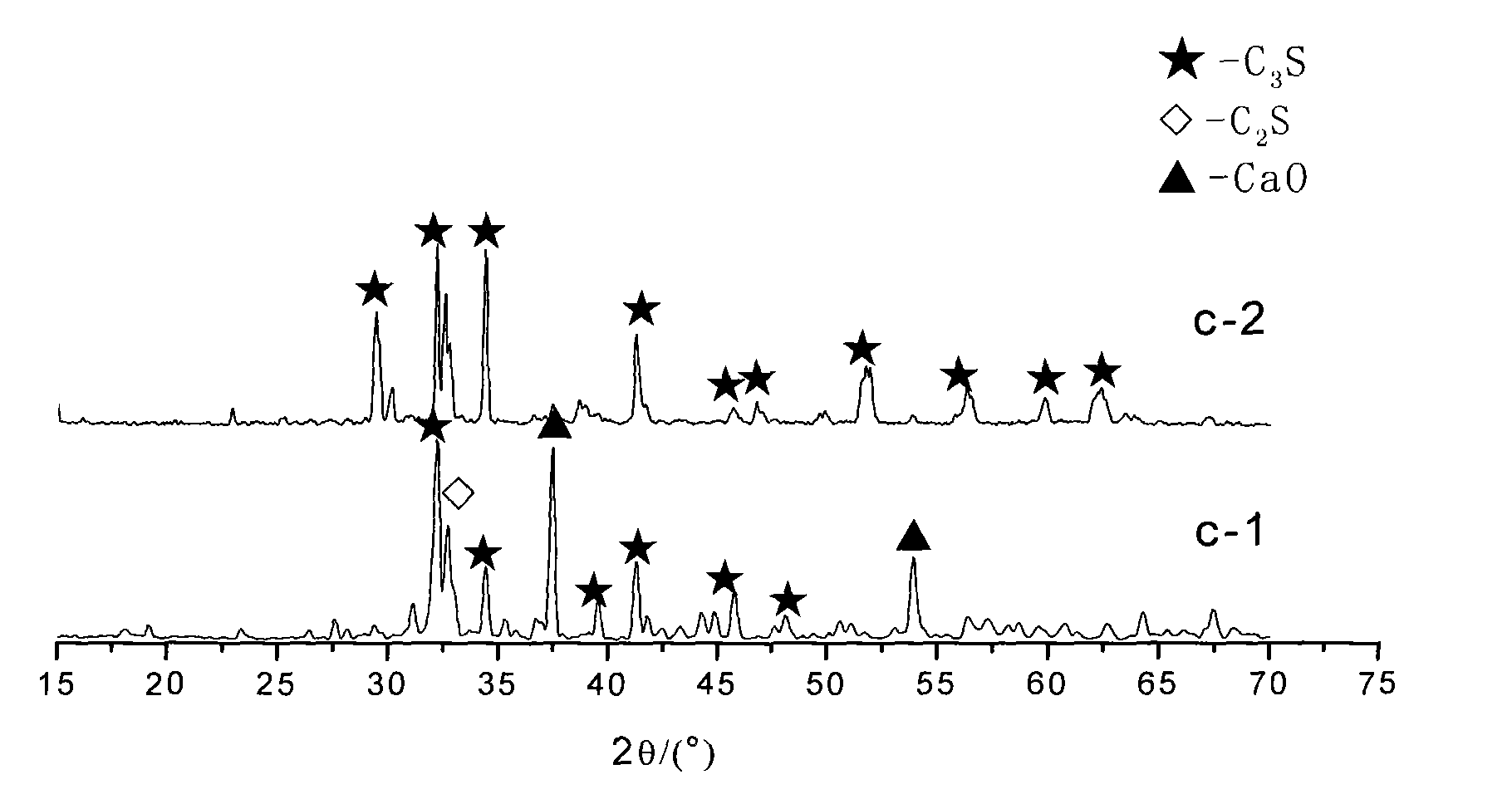
Synthesis technique for high-purity C*S minera
A C3S, high-purity technology, applied in the direction of alkaline earth metal silicate, silicate, etc., can solve the problems of high calcination temperature, less finished products, high calcination temperature, etc., and achieve easy control of reaction conditions, complete solid phase reaction, and easy processing The effect of low temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] 1. Quantity of reactants:
[0023]
[0024] 2. Operation steps:
[0025] Concrete implementation steps of the present invention are as follows:
[0026] 1) Press Ca(NO 3 ) 2 4H 2 The molar ratio of O and ethyl orthosilicate is 3:1 to calculate the amount of reactants required, and the amount of n-propanol required for calculating the molar ratio of n-propanol to ethyl orthosilicate is 5:1, based on water and orthosilicic acid The ethyl ester molar ratio is 2:1 to calculate the amount of water required.
[0027] 2) Use a pipette to measure tetraethyl orthosilicate (AR grade) into a beaker, and take 2.16ml of deionized water from the total water to a beaker containing a certain amount of tetraethyl orthosilicate, and place the beaker under a magnetic force On the stirrer, stir with a magnetic bar and heat to 50°C, stir for 30 minutes to fully hydrolyze the tetraethyl orthosilicate liquid; the measurement is accurate to 0.01ml.
[0028] 3) Weigh the Ca(NO 3 ) 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com