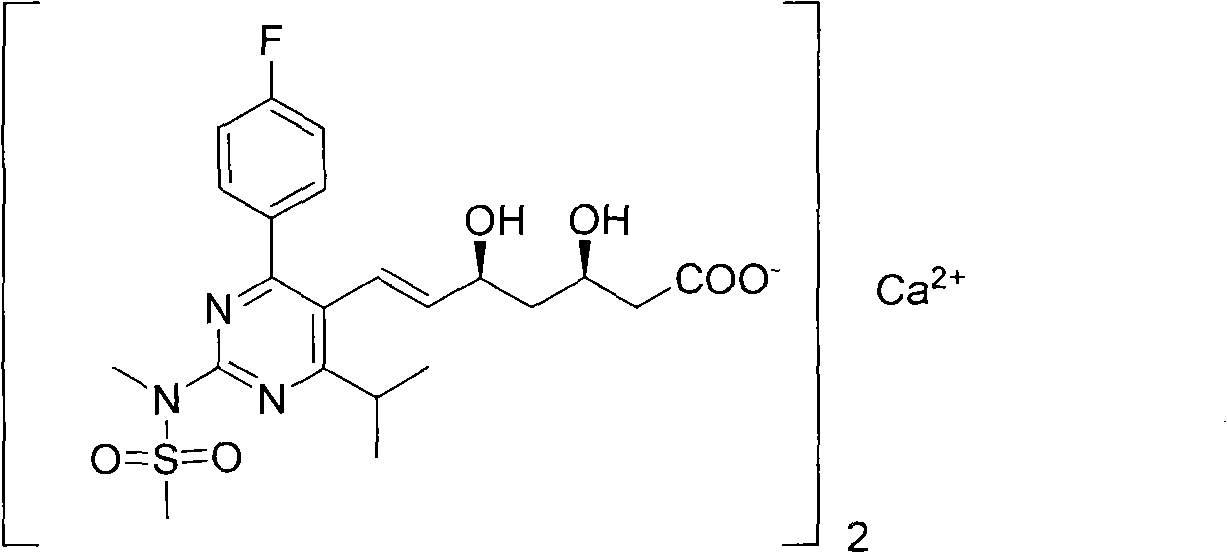
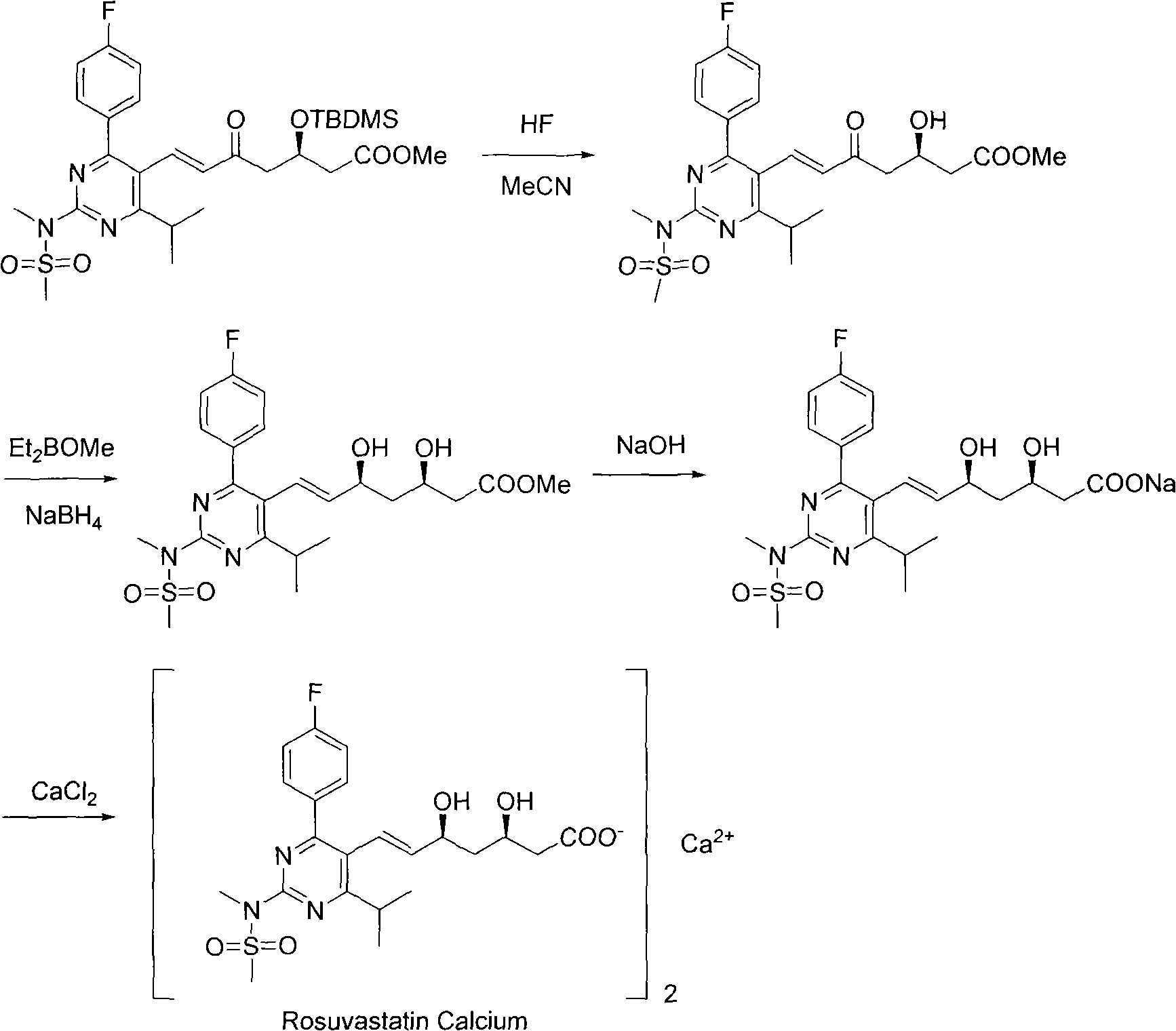
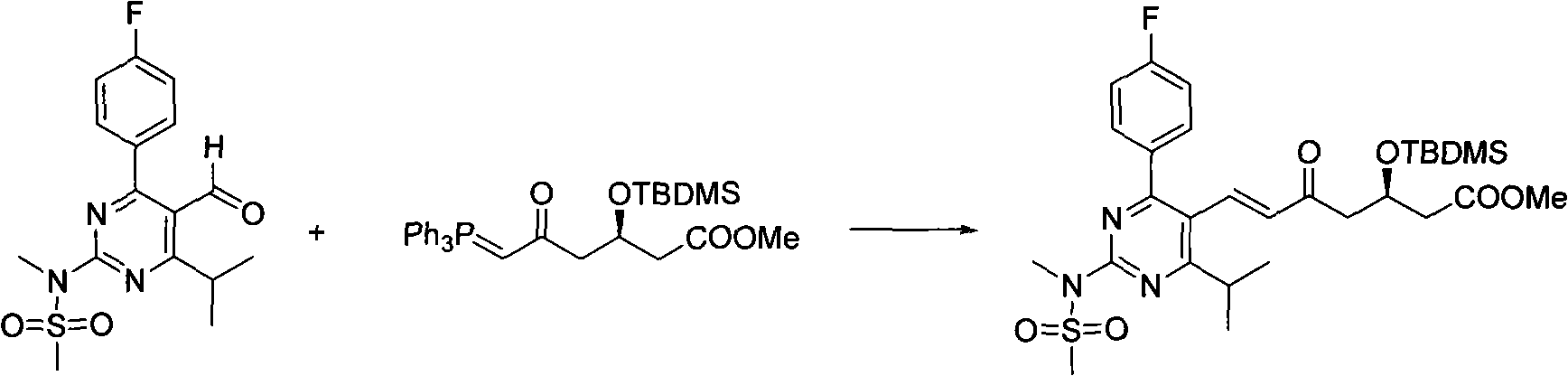
Preparation method for synthesizing intermediate compound of rosuvastatin calcium
A technology for rosuvastatin calcium and intermediates, applied in the field of drug synthesis and chemical engineering, can solve the problems of high production cost, increased cost, high price, etc., and achieve the goal of avoiding the process of column chromatography separation and purification, reducing cost and simplifying steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 500mL three-necked flask equipped with a thermometer and a spherical condenser, add 20.0g (57.01mmol) of N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl ]-N-methylmethanesulfonamide and 30.6g (57.10mmol, 1.0eq) of (3R)-3-(tert-butyldimethylsilyl)oxy-5-carbonyl-6-triphenylphosphine ylide hexanoic acid Methyl ester, N 2 Under protection, 300 mL of acetonitrile was added, and the temperature was raised to 90°C. After stirring under reflux for 24 hours, the temperature was lowered to room temperature, and the acetonitrile was spin-dried under reduced pressure to obtain a crude product.
[0020] Add 80mL of methyl tert-butyl ether, 240mL of n-hexane, 160mL of ethanol, and 160mL of water into the crude product, stir thoroughly for 5 minutes, and separate the liquids (the upper layer is the organic phase, and the lower layer is the aqueous phase). Then add 160mL ethanol and 160mL water into the organic phase, fully stir for 5 minutes, separate the liquid (the u...
Embodiment 2
[0024] In a 500mL three-necked flask equipped with a thermometer and a spherical condenser, add 20.0g (57.01mmol) of N-[4-(4-fluorophenyl)-5-formyl-6-isopropylpyrimidin-2-yl ]-N-methylmethanesulfonamide and 33.7g (62.8mmol, 1.1eq) of (3R)-3-(tert-butyldimethylsilyl)oxy-5-carbonyl-6-triphenylphosphine ylide hexanoic acid Methyl ester, N 2 Under protection, 300 mL of acetonitrile was added, and the temperature was raised to 90°C. After stirring under reflux for 24 hours, the temperature was lowered to room temperature, and the acetonitrile was spin-dried under reduced pressure to obtain a crude product.
[0025] Add 80mL of methyl tert-butyl ether, 240mL of n-hexane, 160mL of ethanol, and 160mL of water into the crude product, stir thoroughly for 5 minutes, and separate the liquids (the upper layer is the organic phase, and the lower layer is the aqueous phase). Then add 160mL ethanol and 160mL water into the organic phase, fully stir for 5 minutes, separate the liquid (the up...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com