Catalyst for decomposing hydrogen sulfide by photocatalysis and preparation method of hydrogen and liquid sulfur by employing same
A catalyst and hydrogen sulfide technology, applied in the field of catalysis, can solve problems such as difficult separation and complex sulfur recovery process, and achieve the effects of high activity, easy realization of reaction conditions, and easy control of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
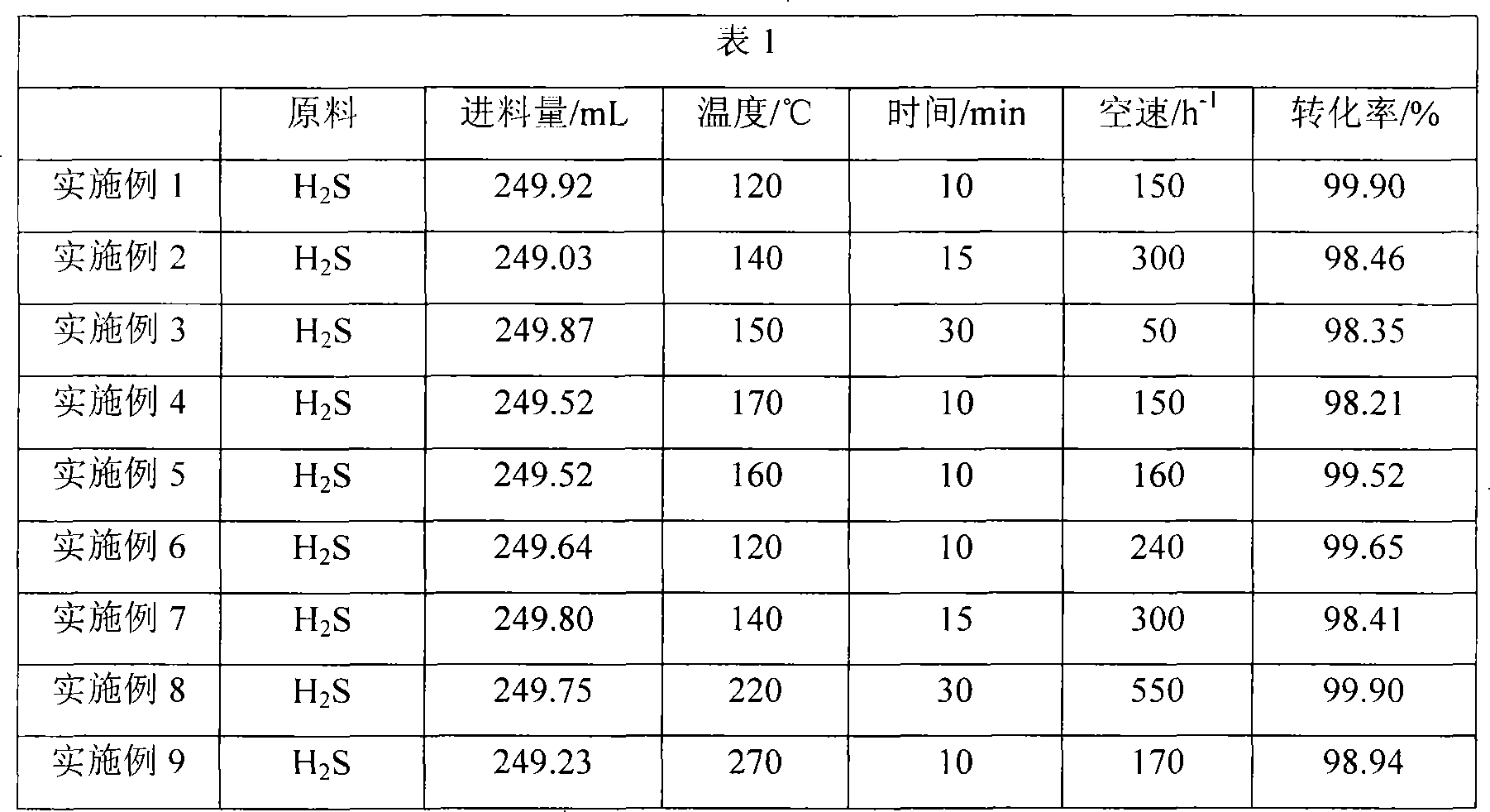
Embodiment 1
[0025] 140g Na 2 Ta 2 o 7 and 44g Ba(OH) 2 Mix and stir at room temperature for 6 hours, and then dry in an oven at 110°C for 6 hours for later use; assuming that the mass percentage of NiO in the finished catalyst is 7.5%, take 0.2027mol Ni(NO 3 ) 2 Prepare a solution, impregnate with the above mixed sample, dry in an oven at 110°C for 6 hours after impregnation, and roast at 650°C for 4 hours to obtain the catalyst required for the reaction. The catalyst was ground to 40 mesh particles as the reaction catalyst. The mass percentage of the carrier in the finally obtained catalyst was 70.3%, the mass percentage of the active component was 22.1%, and the mass percentage of the auxiliary agent was 7.5%.
Embodiment 2
[0027] 50g NH 4 VO 3 and 45g LiOH were mixed and stirred at room temperature for 6 hours, and then dried in an oven at 110°C for 6 hours for later use; Fe in the finished catalyst 2 o 3 Calculated as 5% by mass, take 0.0625mol Fe(OH) 3 Prepare a solution, impregnate with the above mixed sample, dry in an oven at 110°C for 6 hours after impregnation, and roast at 650°C for 4 hours to obtain the catalyst required for the reaction. The catalyst was ground to 80 mesh particles as the reaction catalyst. The mass percent content of the carrier in the finally obtained catalyst is 50%, the mass percent content of the active component is 45%, and the mass percent content of the auxiliary agent is 5%.
Embodiment 3
[0029] 85g NaNbO 3 and 10gNa 2 CO 3 Mix and stir at room temperature for 6 hours, then dry in an oven at 110°C for 6 hours for later use; 3 o 4 Calculated as 5% by mass, take 0.0622mol Co(NO 3 ) 2 Prepare a solution, impregnate with the above mixed sample, dry in an oven at 110°C for 6 hours after impregnation, and roast at 650°C for 4 hours to obtain the catalyst required for the reaction. The catalyst was ground to 60 mesh particles as the reaction catalyst. The mass percent content of the carrier in the finally obtained catalyst is 85%, the mass percent content of the active component is 10%, and the mass percent content of the auxiliary agent is 5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com