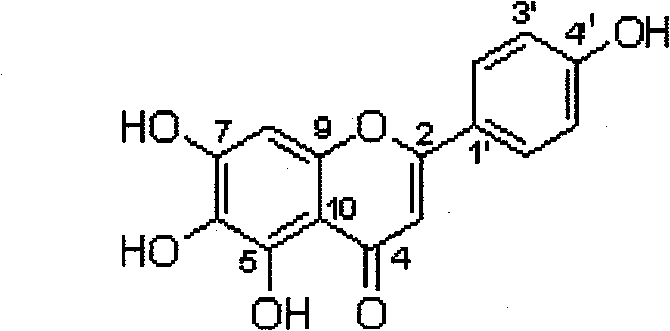
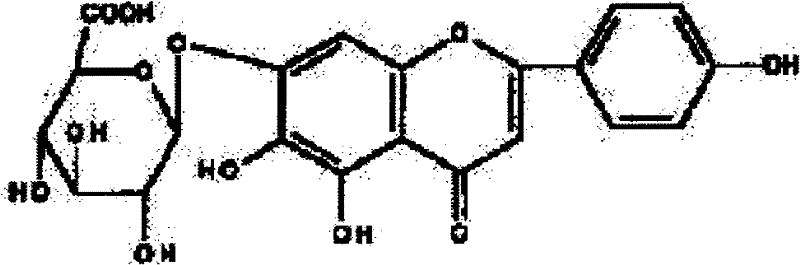
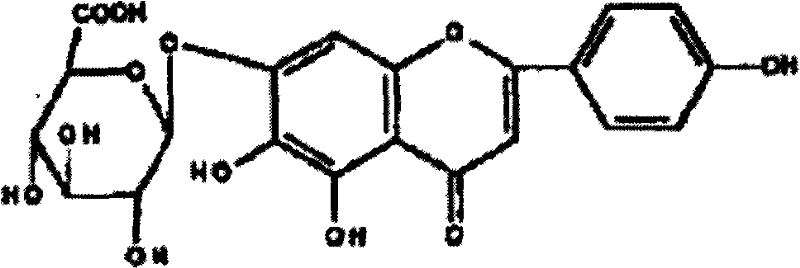
Method for preparing 5,6,7,4'-tetrahydroxyflavone
A kind of technology of tetrahydroxyflavone and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Example 1: Weigh 20.0g of breviscapine raw material, add 500ml of ethylene glycol, 1ml of 4% dilute hydrochloric acid, heat and reflux for 4 hours, filter the product, and dry it to constant weight to obtain 5,6,7,4' -Tetrahydroxyflavone 10.6g, the purity is 96.2% through high performance liquid phase detection. Recrystallize with 60% methanol, filter, and dry to constant weight to obtain 10.1 g of fine product, the purity of which is 98.9% by HPLC.
Embodiment 2
[0142] Example 2: Weigh 15.0g of breviscapine raw material, add 300ml of 1,2-propanediol, 1ml of 5% dilute sulfuric acid, heat and reflux for 3 hours, filter the product, and dry it to constant weight to obtain 5, 6, 7, 7.9g of 4'-tetrahydroxyflavone, the purity is 95.8% by HPLC. Recrystallize with ethanol, filter, and dry to constant weight to obtain 7.1 g of fine product, the purity of which is 99.5% by HPLC.
Embodiment 3
[0143] Example 3: Weigh 12.0 g of scutellarin raw material, add 400 ml of n-butanol, 1 ml of 5% dilute nitric acid, heat and reflux for 5 hours, filter the product, and dry it to constant weight to obtain 5, 6, 7, 4' - 6.5g of tetrahydroxyflavone, the purity of which is 95.7% as detected by high performance liquid phase. Recrystallize with acetone, filter, and dry to constant weight to obtain 5.8 g of fine product, the purity of which is 99.9% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com