Polythiophene compound, intermedium thereof and preparation method and application of same two to solar battery
A technology of polythiophenes and compounds, applied in the direction of organic chemistry, etc., to achieve the effects of correct structure, easy film formation, and improved carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The reaction equation of the method for preparing polythiophenes provided by the invention is as follows:
[0058]
[0059]
[0060] (i) THF, t-BuOK, n-C 6 h 13 Br, room temperature, 12-18h; (ii) ClCH 2 CH 2 Cl, POCl 3 , DMF, reflux, 24-36h; (iii) CHCl 3 , HOAc, NBS, room temperature, 6-12h; (iv) NBS, BPO, CCl 4 , reflux, 4-6h; (v)P(OC 2 h 5 ) 3 , reflux, 1h; (vi)THF, n-BuLi, -78℃, (n-Bu) 3 SnCl, -78°C-room temperature, 24h; (vii) diethyl ether, C 6 h 13 Br, Mg, Ni(dppf)Cl 2 , reflux, 48h;
Embodiment 1
[0062] Embodiment 1, prepare the intermediate of polythiophene compound
[0063] The method for preparing the intermediate of polythiophene compounds provided by the invention comprises the following steps:
[0064] 1) Under the protection of nitrogen, dissolve 0.1 mol of carbazole in 100 mL of tetrahydrofuran, and add 0.2 mol of t-BuOK; under the protection of nitrogen, 0.2 mol of n-C 6 h 13 Br was dissolved in 100mL tetrahydrofuran in another bottle, and then the solution was transferred to a tetrahydrofuran solution containing carbazole, reacted at room temperature for 12 hours, extracted, dried, and recrystallized in methanol to obtain N-hexylcarbazole with a yield of 85%. about;
[0065] Add 0.5mol N-hexylcarbazole prepared above to 1.5mol POCl 3 and 0.525mol of DMF, refluxed for 24 hours, cooled, extracted, dried, and separated by column chromatography to obtain 3-formaldehyde-N-hexylcarbazole (as shown in formula IV), with a yield of about 90%.
[0066] 2) Dissolve ...
Embodiment 2
[0070] Embodiment 2, preparation polythiophene compound
[0071] The method for preparing the polythiophene compounds shown in the general structural formula of formula III provided by the present invention comprises the following steps:
[0072] 1) Under the protection of nitrogen, dissolve 0.2mol of 2,5-dibromothiophene in 150mL of tetrahydrofuran, freeze to -78°C, add 0.5mol of butyllithium, and react at -78°C for 2 hours, then add 0.5mol of 3-Butyltin chloride, slowly warming up to room temperature, reacting for 24 hours, extracting, washing, and vacuum distillation to obtain 2,5-bis(tributyltin-based)thiophene with a yield of about 95%;
[0073] 2) The above-mentioned 2,5-bis(tributyltin-based)thiophene and the intermediate of the polythiophene compound prepared in Example 1 were dissolved in 20ml of toluene in an equimolar ratio (the amount was 1 mmol), and 1% (the Consumption is the catalyst Pd (PPh 3 ) 4 , refluxed for 24-48 hours, cooled, settled in methanol, filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
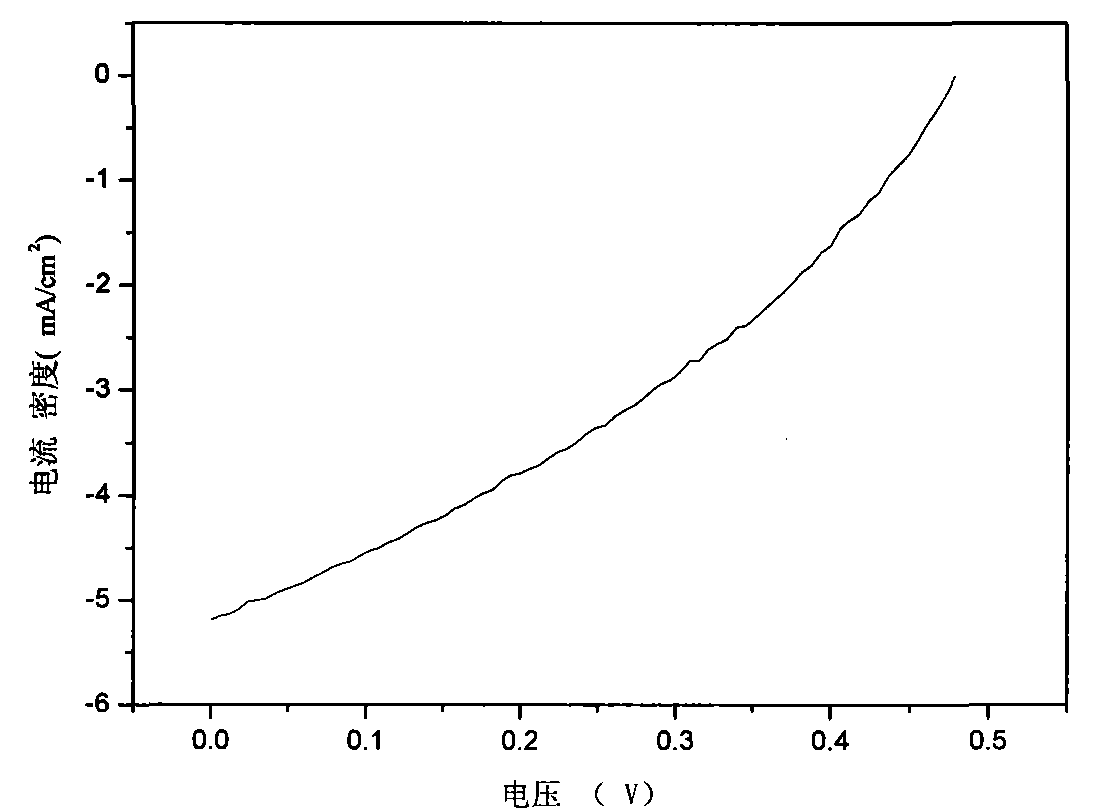
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com