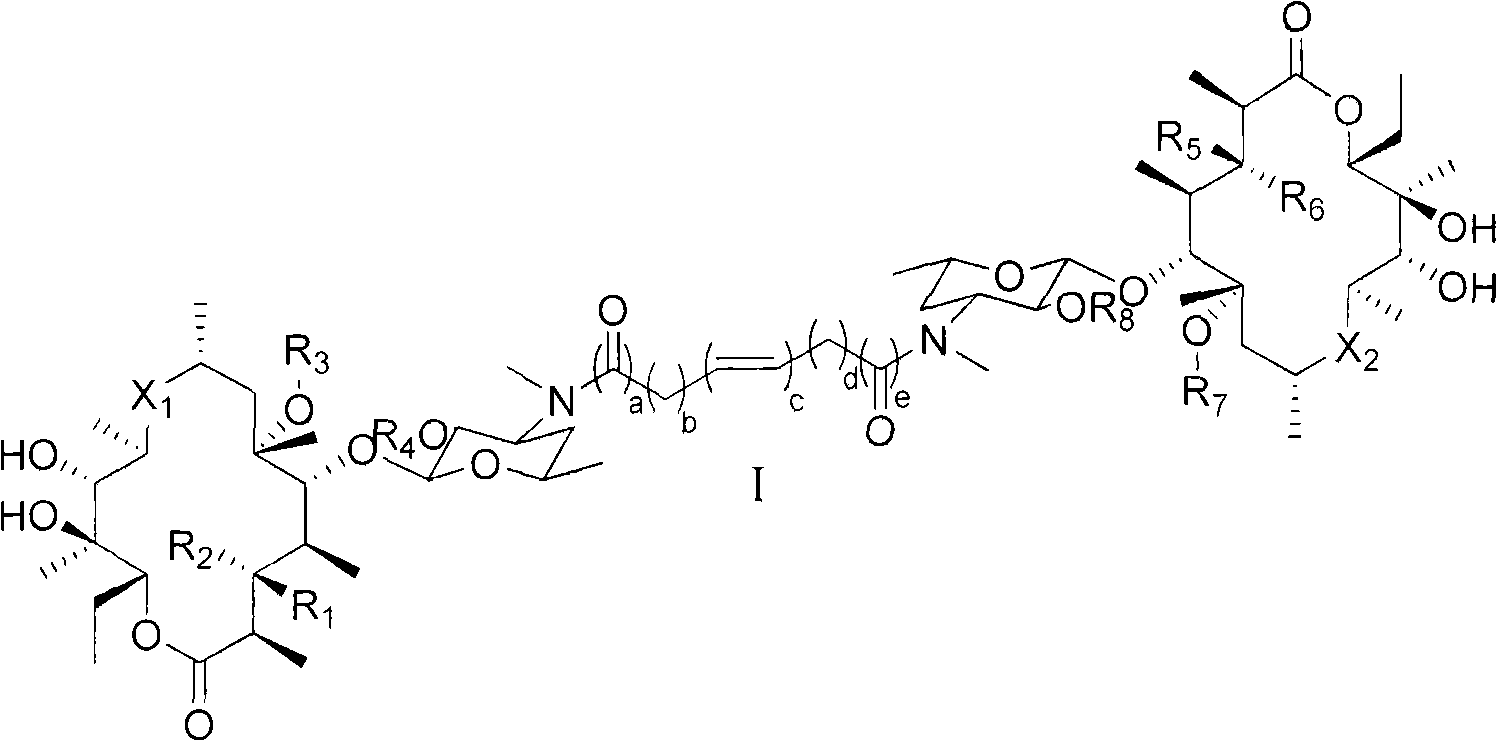
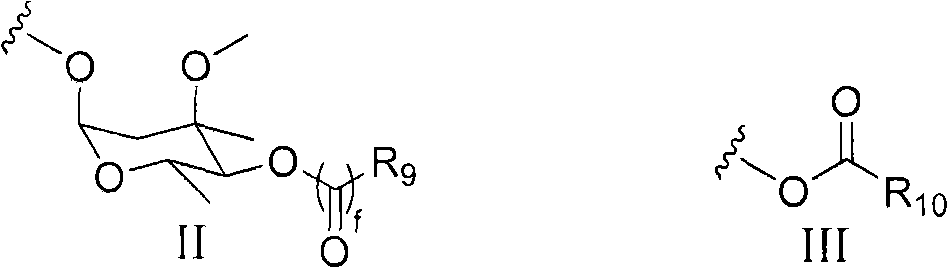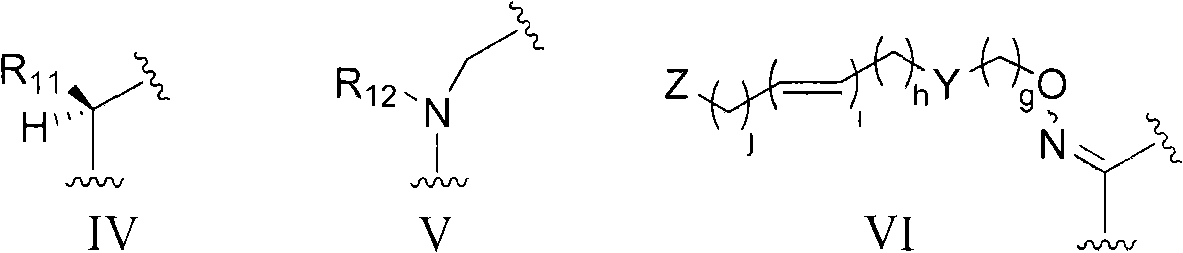Erythromycin derivative and application thereof
A technology of erythromycin derivatives and structural formula, applied in the field of medicine, can solve the problem that the activity of erythromycin derivative tumor cell proliferation has not been reported in literature and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Embodiment 1: the preparation of compound 1
[0153] Step A
[0154] Dissolve erythromycin (6.00 g, 8.18 mmol) in 100 mL of methanol, then add NaBH 4 (0.93g, 24.54mmol), stirred at room temperature for 24h. Then 10 mL of distilled water was added and methanol was distilled off, then the solution was poured into water and extracted with chloroform, washed with water and dried over anhydrous sodium sulfate. The solution was then concentrated and evaporated to dryness, and the resulting crude product was purified by silica gel column chromatography (chloroform:methanol:ammonia=10:0.5:0.01~10:1:0.05) to obtain 4.38g of 9(S)-hydroxyerythromycin in a yield of 70 %.
[0155] Step B
[0156] The product obtained in step A (3.00 g, 4.08 mmol), sodium acetate (2.80 g, 20.4 mmol) were dissolved in 100 mL of methanol and 20 mL of water. Then iodine (1.24 g, 4.90 mmol) was added, the reaction was stirred at 50°C for 6 h, and the pH of the solution was controlled to 8.0-9.0 with...
Embodiment 2
[0159] Embodiment 2: the preparation of compound 2
[0160] Using roxithromycin as a raw material, the title compound was prepared in the same manner as steps B and C in Example 1, with a yield of 88%; MS 837.4[M / 2+H] + ; compound 13 See Table-1 for C-NMR.
Embodiment 3
[0161] Embodiment 3: the preparation of compound 3
[0162] Step A
[0163] Erythromycin (12.00g, 16.36mmol), triethylamine (5.71mL, 40.90mmol) and hydroxylamine hydrochloride (5.70g, 81.98mmol) were dissolved in 12mL of methanol, stirred and refluxed for 24h. Then the reaction solution was cooled to 0° C., at which time a solid precipitated out, which was filtered and the filter cake was washed twice with cold methanol. Dissolve the solid in 10 mL of methanol, add 4 mL of ammonia water to the solution, stir for 30 minutes, and then add 4 mL of distilled water. Subsequently, crystals were precipitated, and 10 mL of distilled water was added, filtered with suction, and dried to obtain 6.36 g of 9(E)-erythromycin oxime as a white solid, with a yield of 52%.
[0164] Step B
[0165] The product obtained in Step A (0.85 g, 1.13 mmol) was dissolved in 10 mL of anhydrous ether and 1 mL of DMF, and NaH (95%) (42.69 mg, 1.69 mmol) was added to the solution. When no more gas was ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com