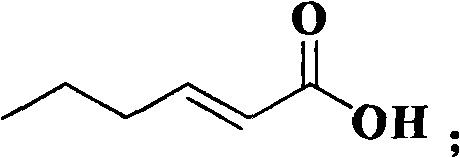
Method for synthesizing (E)-2-hexenoic acid
A synthesis method and technology of hexenoic acid, applied in chemical instruments and methods, preparation of carboxylic acid by ozone oxidation, organic compound/hydride/coordination complex catalyst, etc., can solve harsh reaction conditions, use of toxic solvents, side reactions Serious problems, to achieve the effect of less side reactions, mild reaction conditions and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 10 ml of water, 5.0 mmoles of diethyl malonate, 5.0 mmoles of n-butyraldehyde and 0.15 mmoles of catalyst TBAB to a reaction flask equipped with a reflux condenser and a stirrer. The reaction mixture was stirred at 25°C for 12 hours. After that, the temperature was raised to 60°C, and the stirring was continued for 6.0 hours, and then the temperature was raised to 90°C, and the stirring was continued for 2 hours, then 10% by mass sodium hydroxide solution was added, stirred and refluxed for 0.5 hours, and then 10% by mass hydrochloric acid was added to acidify , Stirring and refluxing for 0.5 hours, stopping the reaction, distillation under reduced pressure, and vacuum drying to obtain 0.485 g of (E)-2-hexenoic acid with a yield of 85%.
[0020] The reaction equation is as follows:
[0021]
[0022] Data characterization of hexenoic acid:
[0023] Mp: 125~127℃ / 2.66KPa
[0024] 1 H NMR(500MHz, CDCl 3 , 25℃)δ=0.87(3H), 1.41(2H), 2.14(2H), 5.76(2H), 6.80(1H), 12.14(1H). ...
Embodiment 2
[0029] The dosage of n-butyraldehyde was 0.48 mmol, and other operating conditions were the same as in Example 1. The yield of (E)-2-hexenoic acid was 82%, 124-125°C / 2.66KPa.
Embodiment 3
[0031] Except that the dosage of n-butyraldehyde is 0.52 mmol, other operating conditions are the same as in Example 1, and the yield of (E)-2-hexenoic acid is 80%, 125-126°C / 2.66KPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com