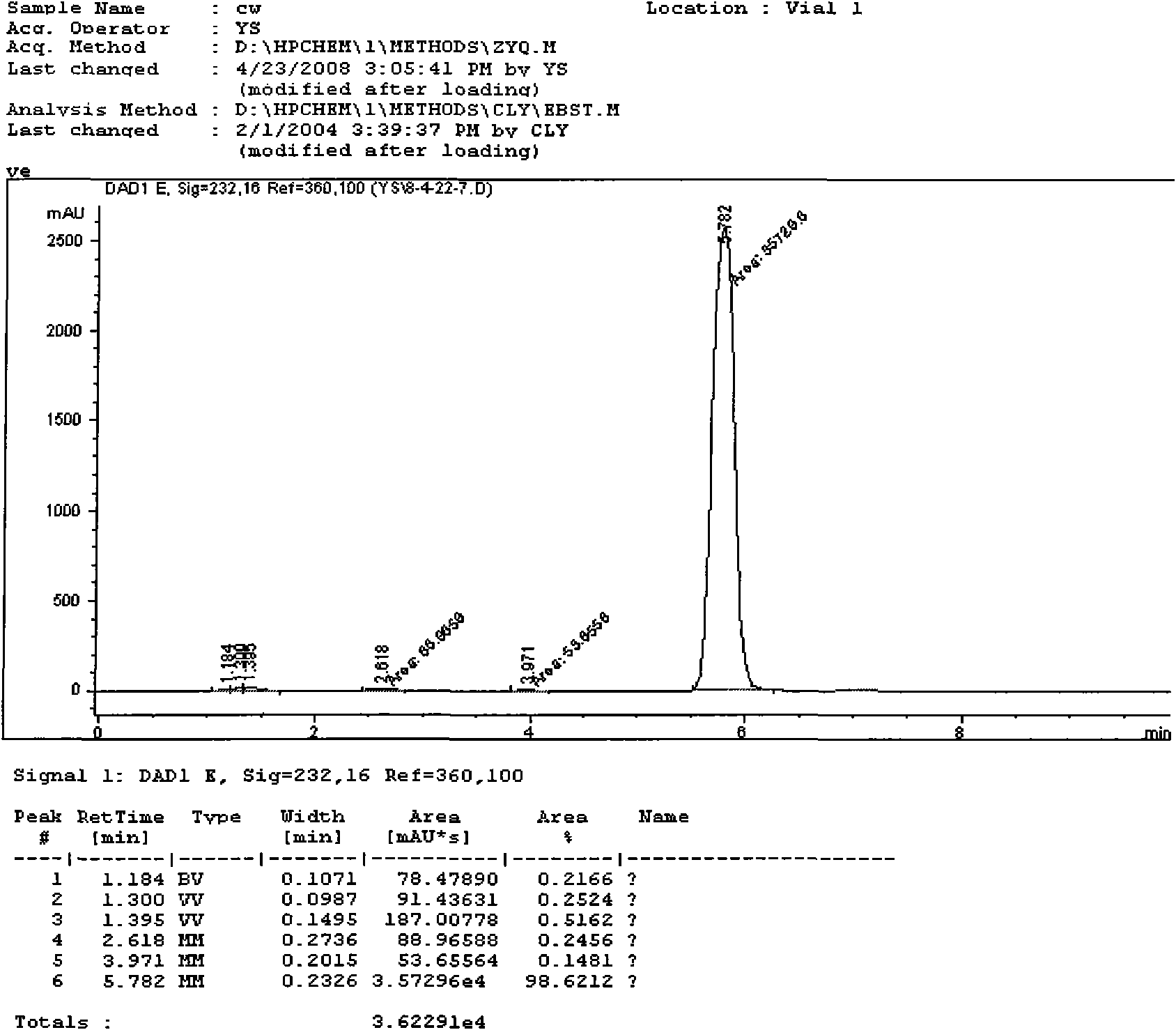
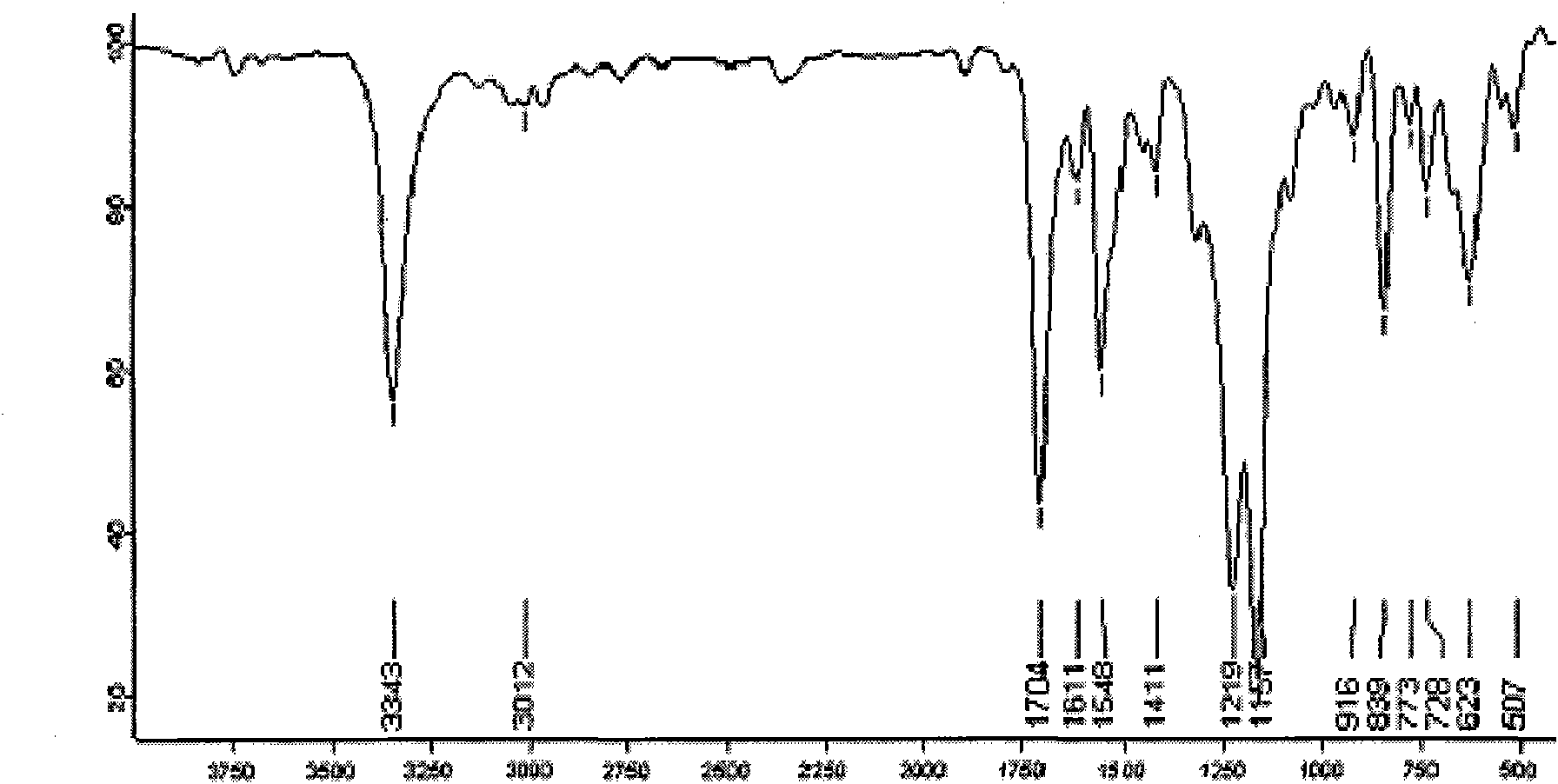
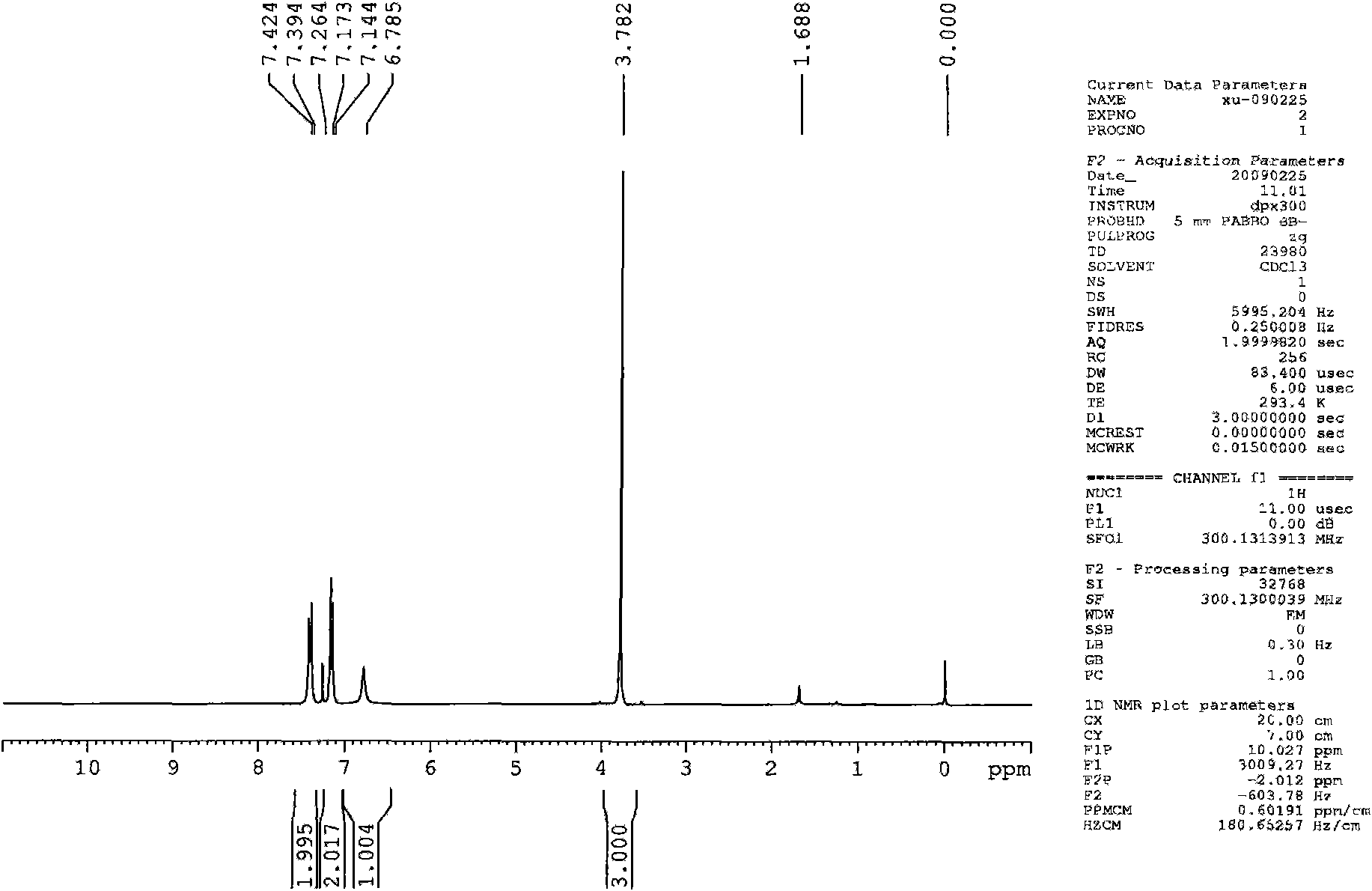
Method for preparing 4-trifluoro-methoxy phenyl carbamate
A technology of methyl carbamate and trifluoromethoxybenzene, which is applied in the field of preparation of methyl 4-trifluoromethoxyphenyl carbamate, can solve problems such as not meeting the requirements of green chemistry, and achieves simple operation, good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under normal pressure, first add 10.80mL (0.12mol) of dimethyl carbonate and 0.8856g of zinc acetate catalyst to a 50mL three-necked flask, connect the flask to a condensation reflux column, and feed N into the system. 2 , stirred magnetically and raised the temperature to about 170°C and maintained it for a period of time, then added 2.71mL (0.02mol) of 4-trifluoromethoxyaniline and raised the temperature to 180°C, then started timing. where n (4-三氟甲氧基苯胺) : n (碳酸二甲酯) =1:6. After reacting for 10 hours, let it stand for 2 hours, and the filtrate was washed with 15mL CH 2 Cl 2 After refluxing at 55°C for a period of time, extraction was carried out, and the extract was left to stand at room temperature for 24 hours, then spin-dried, and then further purified by column chromatography (300-400 mesh silica gel as a stationary phase), and then extracted with petroleum ether and ethyl acetate. Ester as eluent (V (石油醚) :V (乙酸乙酯) =3:1), a pale yellow solid was isolated wit...
Embodiment 2
[0030] Under normal pressure, first add 7.20mL (0.08mol) of dimethyl carbonate and 0.8856g of zinc acetate catalyst into a 50mL three-necked flask, connect the flask to a condensation reflux column, and pass people N into the system. 2 , stirred magnetically and raised the temperature to about 170°C and maintained it for a period of time, then added 2.71mL (0.02mol) of 4-trifluoromethoxyaniline and raised the temperature to 180°C, then started timing. where n (4-三氟甲氧基苯 胺) : n (碳酸二甲酯) =1:4. After reacting for 7 hours, 3.60 mL (0.04 mol) of dimethyl carbonate was added to make the molar ratio of 4-trifluoromethoxyaniline to dimethyl carbonate finally 1:6. Continue to react until 10 hours to stop the reaction, let it stand for 2 hours, and the filtrate is washed with 15mL CH 2 Cl 2 After refluxing at 55°C for a period of time, extraction was carried out, and the extract was left to stand at room temperature for 24 hours, then spin-dried, and then further purified by column c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com